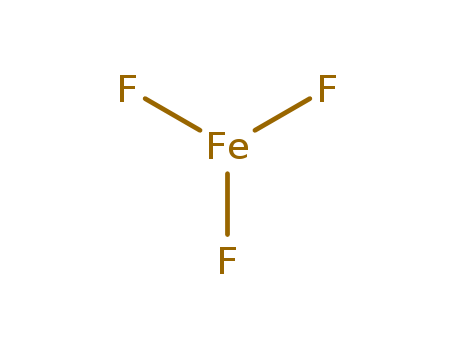- Molecular Formula: F3Fe
- CasNo.: 7783-50-8
- Melting point: 1000 °C
- Appearance: green powder
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
High Purity Factory Supply High Purity anhydrous 99.99% Iron Fluoride 7783-50-8 with the Best Price
- Molecular Formula:F3Fe
- Molecular Weight:112.842
- Appearance/Colour:green powder
- Vapor Pressure:922mmHg at 25°C
- Melting Point:1000 °C
- Boiling Point:19.5 °C at 760 mmHg
- PSA:0.00000
- Density:3.87 g/cm3
- LogP:1.25810
Iron(III) fluoride(Cas 7783-50-8) Usage
General Description
Iron(III) fluoride is a chemical compound with the formula FeF3. It is a greyish-white solid that is highly soluble in water. Iron(III) fluoride is commonly used as a precursor for the preparation of other iron compounds and as a catalyst in chemical reactions. It is also used in the manufacturing of reagents, pharmaceuticals, and in the production of fluorides. Iron(III) fluoride has also been investigated for its potential applications in dental care and as a potential treatment for iron deficiency. It is important to handle iron(III) fluoride with care as it is toxic if ingested or inhaled, and proper safety procedures should be followed when working with this compound.
InChI:InChI=1/2FH.Fe/h2*1H;/q;;+2/p-2
7783-50-8 Relevant articles
Reactions of oxides of some 3d elements with ammonium hydrogen difluoride
Kalinnikov,Nesterov,Makarov,Steshin,Tikhomirova
, p. 347 - 352 (2004)
Reactions of iron, manganese(II), mangan...
The first oligomeric anions of fluoro-litho metallates with octahedra sandwich motive: Cs4K{[F3MIIIF3]Li[F 3MIIIF3]}, MIII = Ga, Fe
Bork,Hoppe
, p. 297 - 307 (1996)
Colourless single crystals of Cs4K{Li[Ga...
Moessbauer spectroscopy and magnetic properties of Ba5Feiii3-xMii19-x (M = Fe, Cu)
Gredin, Patrick,De Kozak, Ariel,Pierrard, Angelique,Calage, Yvon
, p. 159 - 164 (1996)
The Moessbauer spectra of three phases o...
Enhancing cycling performance of FeF3 cathode by introducing a lightweight high conductive adsorbable interlayer
Zhou, Xiangyang,Sun, Hongxu,Zhou, Haochen,Xu, Zhanglin,Yang, Juan
, p. 317 - 326 (2017)
Iron fluorides, as a kind of high specif...
Structure and electrochemical performance of FeF3/V2O5 composite cathode material for lithium-ion battery
Wu, Wen,Wang, Ying,Wang, Xianyou,Chen, Quanqi,Wang, Xin,Yang, Shunyi,Liu, Xiuming,Guo, Jia,Yang, Zhenhua
, p. 93 - 96 (2009)
Orthorhombic structure FeF3 was synthesi...
MOSSBAUER SPECTROSCOPY STUDY OF CRYSTALLIZATION OF AMORPHOUS IRON (III) FLUORIDES: INFLUENCE OF EXPERIMENTAL CONDITIONS.
Greneche, J. M.,Varret, F.,Leblanc, M.,Ferey, G.
, p. 813 - 816 (1987)
Isothermal and non-isothermal treatments...
Synthesis and thermal decomposition of (NH4)2[MIIIF5(H2O)] (M = Al, Fe and Cr)
Bentrup. U.,Kolditz, L.
, p. 827 - 832 (1988)
Ammonium pentafluorometallate monohydrat...
mer-Triammine trifluorido iron(III), mer-[FeF3(NH 3)3]
Kraus, Florian,Baer, Sebastian A.
, p. 865 - 867 (2011)
Metal fluorides are scarcely soluble in ...
Characterization of an Amorphous Change of FeF3: Thermal, Magnetic and Mossbauer Study.
Ferey,Leclerc,de Pape,Mariot,Varret
, p. 477 - 480 (1979)
Amorphous FeF//3 has been prepared by fa...
An electron diffraction and crystal chemical investigation of oxygen/flourine ordering in rutile-type iron oxyflouride, FeOF
Brink, Frank J.,Withers, Ray L.,Thompson, John G.
, p. 359 - 365 (2000)
Rutile-type iron oxyfluoride, FeOF, has ...
Magnetic structure and properties of Pb8FeIIFe2IIIF24: A 1-D ferrimagnetic chain compound exhibiting a spin-flop transition
Pierrard,Gredin,Dupont,De Kozak,Bouree-Vigneron,Andre,Rosenman
, p. 44 - 51 (1999)
The magnetic structure of Pb8FeIIFe2IIIF...
On the crystal structure of pyrochlores: Moessbauer spectra of orthorhombic CsFe2F6 and X-ray single crystal studies of the cubic compounds CsMgGaF6, CsMIIVIIIF 6 (MII = Mn, Zn), CsMIIFeIIIF 6 (MII = Mn, Cu, Zn), and Cs4Cu 5V3O ...
Baum, Elke,Dahlke, Patrik,Kaiser, Volker,Molinier, Michel,Schmidt, Roland E.,Pebler, Juergen,Massa, Werner,Babel, Dietrich
, p. 2244 - 2250 (2008/10/09)
Title full: On the crystal structure of ...
7783-50-8 Process route
-
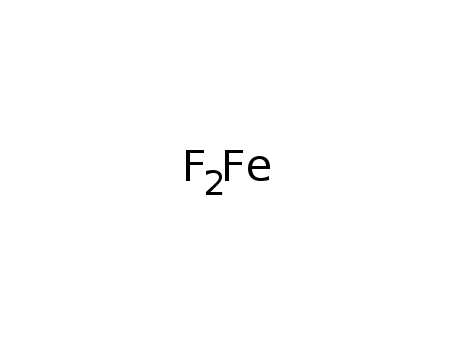
-
iron(II) fluoride

-
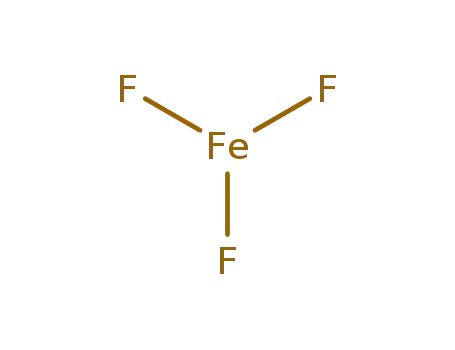
- 7783-50-8
iron(III) fluoride

-
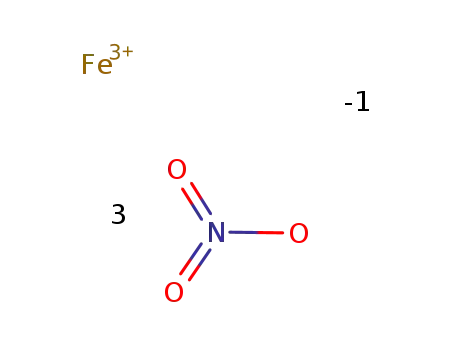
- 7782-61-8
ferric nitrate
ConditionsConditions Yield With nitric acid; In water; discoloration of the aq. soln. of FeF2 with HNO3; formation of FeF3 and Fe(NO3)3;; pptn. as a solid mixt. on evapn.;;With HNO3; In water; discoloration of the aq. soln. of FeF2 with HNO3; formation of FeF3 and Fe(NO3)3;; pptn. as a solid mixt. on evapn.;;-
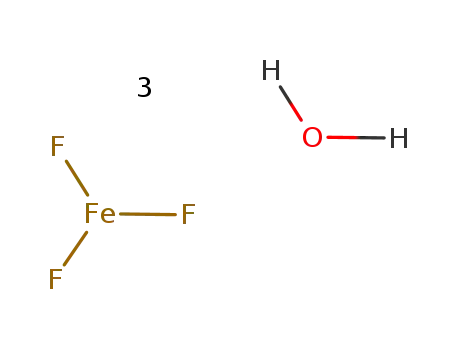
-
iron(III) fluoride trihydrate

-

- 7783-50-8
iron(III) fluoride
ConditionsConditions Yield In neat (no solvent); byproducts: H2O; under HF stream, 750°C;In neat (no solvent); at 120 ℃; for 48h;7783-50-8 Upstream products
-
7664-39-3
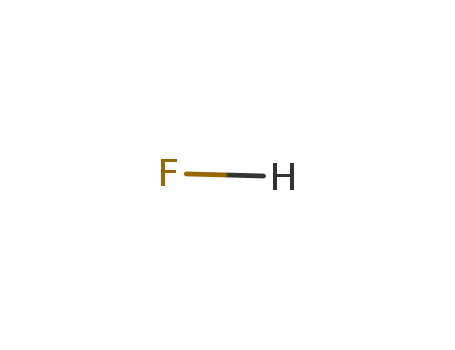
hydrogen fluoride
-
7782-50-5
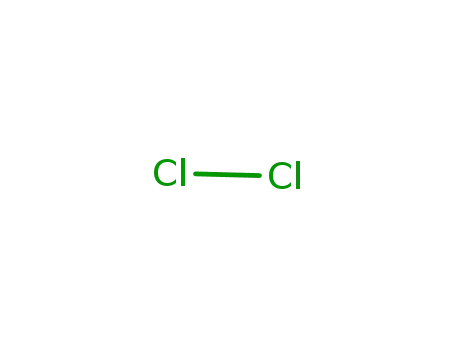
chlorine
-
7439-89-6
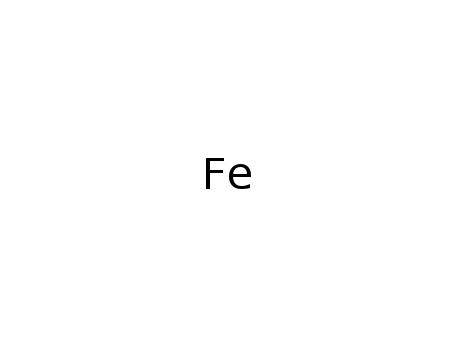
iron
-
7782-41-4
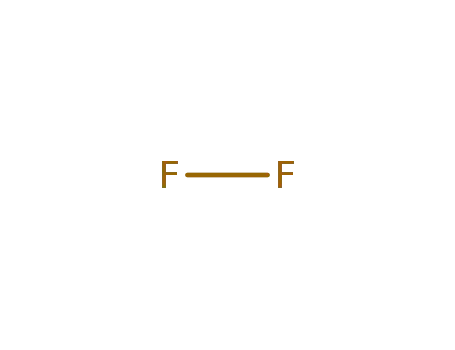
fluorine
7783-50-8 Downstream products
-
7705-08-0
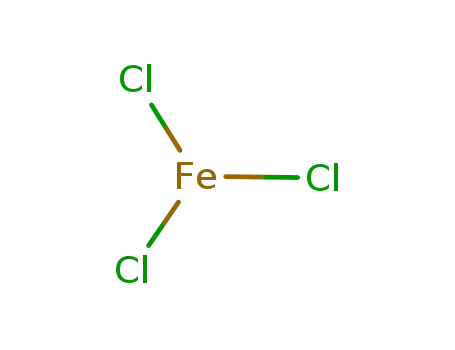
iron(III) chloride
-
7637-07-2
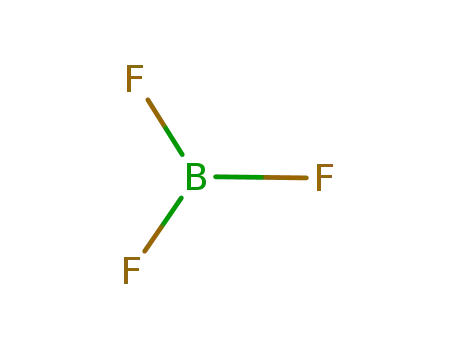
boron trifluoride
-
7664-39-3
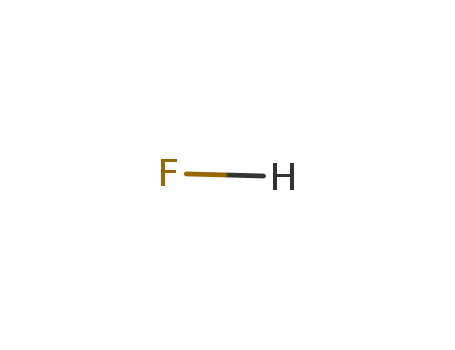
hydrogen fluoride
-
7439-89-6
iron