- Molecular Formula: Bi
- CasNo.: 7440-69-9
- Melting point: 271 °C(lit.)
- Appearance: silver-grey or reddish metal, or black powder
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
Buy High Grade Quality Factory Supply 99.999%-99.9999% Bismuth shot 7440-69-9 with Safe Shipping
- Molecular Formula:Bi
- Molecular Weight:208.98
- Appearance/Colour:silver-grey or reddish metal, or black powder
- Vapor Pressure:<0.1 mm Hg ( 20 °C)
- Melting Point:271 °C(lit.)
- Boiling Point:1560 °C
- PSA:0.00000
- Density:9.8 g/cm3
- LogP:0.33750
- IDLH: N.D.See: IDLH INDEX
Bismuth(Cas 7440-69-9) Usage
Isotopes
There are a total of 59 radioactive isotopes for bismuth, ranging in half-livesfrom a few milliseconds to thousands of years. At one time it was thought that there wasjust one stable isotope (Bi-209), but it was later found that Bi-209 is radioactive witha half-life of 19,000,000,000,000,000,000 years. Such a long half-life means that Bi-209 has not completely disintegrated and is still found in nature, and is thus consideredstable. In this case, Bi-209 makes up 100% of Bismuth’s natural abundance.
Origin of Name
Bismuth was known and used by the ancient alchemists along with other metals both for chemical reactions and for medical purposes. The name comes from the German bismu, which had been changed from wismu, meaning “white.”
Characteristics
Bismuth is more resistant to electrical current in its solid state than it is in its liquid form.Its thermal conductivity is the lowest of all metals, except mercury. Even though it is considereda metal-like element, it is a very poor conductor of heat and electricity.Bismuth has a characteristic similar to water. It expands when changing from the liquidphase to the solid phase. This factor makes it useful as an alloy in metals that are used to fillmolds, given that it will expand to the cast’s dimensions.
History
In early times bismuth was confused with tin and lead. Claude Geoffroy the Younger showed it to be distinct from lead in 1753. It is a white crystalline, brittle metal with a pinkish tinge. It occurs native. The most important ores are bismuthinite or bismuth glance (Bi2S3) and bismite (Bi2O3). Peru, Japan, Mexico, Bolivia, and Canada are major bismuth producers. Much of the bismuth produced in the U.S. is obtained as a by-product in refining lead, copper, tin, silver, and gold ores. Bismuth is the most diamagnetic of all metals, and the thermal conductivity is lower than any metal, except mercury. It has a high electrical resistance, and has the highest Hall effect of any metal (i.e., greatest increase in electrical resistance when placed in a magnetic field). “Bismanol” is a permanent magnet of high coercive force, made of MnBi, by the U.S. Naval Surface Weapons Center. Bismuth expands 3.32% on solidification. This property makes bismuth alloys particularly suited to the making of sharp castings of objects subject to damage by high temperatures. With other metals such as tin, cadmium, etc., bismuth forms low-melting alloys that are extensively used for safety devices in fire detection and extinguishing systems. Bismuth is used in producing malleable irons and is finding use as a catalyst for making acrylic fibers. When bismuth is heated in air it burns with a blue flame, forming yellow fumes of the oxide. The metal is also used as a thermocouple material, and has found application as a carrier for U235 or U233 fuel in atomic reactors. Its soluble salts are characterized by forming insoluble basic salts on the addition of water, a property sometimes used in detection work. Bismuth oxychloride is used extensively in cosmetics. Bismuth subnitrate and subcarbonate are used in medicine. Natural bismuth contains only one isotope 209Bi. Forty-four isotopes and isomers of bismuth are known. Bismuth metal (99.5%) costs about $250/kg.
Production Methods
Bismuth is obtained as a by-product in smelting and refining of lead, copper or tungsten ores. The metal is partially volatilized when the ore is smelted at the high temperature. Separation from copper is achieved by electrolytic refining, bismuth accumulating in the anode slimes with lead, arsenic, antimony, tellurium, and other metal impurities. All throughout the smelting and refining operations bismuth accompanies lead. It finally is removed from lead by Betterton-Kroll or Betts processes. The Betterton-Kroll process involves the addition of calcium-lead alloy or magnesium metal to lead slime, thus converting bismuth to high-melting bismuthides of calcium or magnesium, Ca3Bi2 or Mg3Bi2, respectively. These bismuthides liquate from the bath and are separated as dross. Bismuth dross is then melted in kettles forming Bi7Mg6K9 which liquates to the top of the bath and is removed from the molten lead. Treatments with caustic soda finally produce the high quality bismuth.In a modified process, potassium substitutes for calcium to form Bi7Mg6Ca9 which liquates to the top of the bath and is removed from the molten lead. The Betts process is based on electrolytic refining using a solution of lead fluorosilicate and fluorosilicic acid. While lead is deposited on the cathode, bismuth goes to the anode where it is collected with other impurity metals. It is then filtered, dried, smelted, and further refined, depending on the purity desired. Impurities are removed by adding molten caustic and zinc, and finally by chlorination.Bismuth may be obtained from other ores, too. The recovery process however, depends primarily on the chemical nature of the ores. For example, the sulfide ore requires smelting, carbon reduction, and the addition of iron (to decompose any bismuth sulfide present). Oxide ores, on the other hand, are treated with hydrochloric acid to leach bismuth from the mineral. The bismuth chloride solution is then diluted with water to precipitate bismuth oxy-chloride. The precipitate is roasted with lime and charcoal. Satisfactory recovery of the metal from its carbonate ore may be achieved by both the above techniques.Bismuth is sold in the form of rod, lump, powder, and wire.
Hazard
Bismuth is flammable as a powder. The halogen compounds of bismuth are toxic wheninhaled or ingested. Some of the salts of bismuth can cause metallic poisoning in a mannersimilar to mercury and lead.At the beginning of the twentieth century, before penicillin, bismuth compounds wereused to treat some venereal diseases. However, the treatment was generally unsuccessful.
Health Hazard
Exposures to bismuth salts are associated primarily by ingestion. Bismuth is known to cause adverse health effects. The symptoms include, but are not limited to, irritation of the eyes, skin, respiratory tract, lungs, foul breath, metallic taste, and gingivitis. On ingestion, bismuth causes nausea, loss of appetite, weight, malaise, albuminuria, diarrhea, skin reactions, stomatitis, headache, fever, sleeplessness, depression, rheumatic pain, and a black line may form on gums in the mouth due to deposition of bismuth sulfi de. Prolonged exposure to bismuth causes mild but deleterious effects on the kidneys and high concentrations of bismuth result in fatalities. Occupational exposures to bismuth occur during the manufacture of cosmetics, industrial chemicals, and pharmaceuticals. Acute exposure with over dosage of bismuth-containing drugs causes anorexia, nausea, vomiting, abdominal pain, and possibly a dry mouth and thirst. Bismuth also causes neurotoxicity. Bismuth pentafl uoride is highly toxic and causes irritation to the skin, eyes, and respiratory tract, while bismuth subnitrate causes blurred vision.
Flammability and Explosibility
Nonflammable
Potential Exposure
Bismuth is used as a constituent of tempering baths for steel alloys; in low Freezing/Melting point alloys which expand on cooling; in aluminum and steel alloys to increase machinability; and in printing type metal. Bismuth compounds are found primarily in pharmaceuticals as antiseptics, antacids, antiluetics, and as a medicament in the treatment of acute angina. They are also used as a contrast medium in roentgenoscopy and in cosmetics. For the general population the total intake from food is 5 20 μg with much smaller amounts contributed by air and water.
Carcinogenicity
An old lifetime study with rats fed 2% bismuth subcarbonate (BSC) in the diet did not show an increase of tumors or a decrease of survival.
Environmental Fate
The mechanism by which bismuth produces toxicity has not been identified. Interaction with thiol compounds has been proposed as a primary mechanism.
Shipping
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1—Flammable solid.
Purification Methods
Melt it in an atmosphere of dry helium, then filter through dry Pyrex wool to remove any bismuth oxide present [Mayer et al. J Phys Chem 64 238 1960].
Toxicity evaluation
In aerated water, bismuth oxidizes; however, in an anaerobic aqueous environment, bismuth is unaffected. Similarly, in the atmosphere, bismuth is unaffected unless condensation or deposition of water occurs. Due to the inability for air and water to affect bismuth under most circumstances, bismuth tends to persist until wet or dry deposition, and therefore long-range transport is possible and likely.
Incompatibilities
Finely divided powder is highly flammable. Reacts with strong acids and strong oxidizers, chlorine, fused ammonium nitrates, iodine pentafluoride, and nitrosyl fluoride.
Waste Disposal
Dissolve in a minimum amount of concentrated HCl. Dilute with water until precipitate is formed. Redissolve in HCl. Then saturate with H2S. Filter, wash, dry and return to supplier.
Physical properties
Bismuth has unusually low toxicity for a heavy metal. Bismuth is stable to both dry and moist air at ordinary temperatures. When red hot, it reacts with water to make bismuth(III) oxide. Bismuth forms trivalent and pentavalent compounds. The trivalent compounds are more common. Many of its chemical properties are similar to those of As and Sb, although they are less toxic than derivatives of those lighter elements. At elevated temperatures, the vapors of the metal combine rapidly with oxygen, forming the yellow trioxide, Bi2O3.
Definition
A brittle pinkish metallic element belonging to group 15 (formerly VA) of the periodic table. It occurs native and in the ores Bi2S3 and Bi2O3. The element does not react with oxygen or water under normal temperatures. It can be dissolved by concentrated nitric acid. Bismuth is widely used in alloys, especially low-melting alloys. The element has the property of expanding when it solidifies. Compounds of bismuth are used in cosmetics and medicines.
General Description
All foils are mounted on a permanent support which cannot be removed without damaging the foil.
Industrial uses
Bismuth (symbol Bi) is a brittle, crystallinemetal with a high metallic luster with a distinctivepinkish tinge. The metal is easily cast butnot readily formed by working. Within a narrowrange of temperature, around 225°C, it can beextruded. Its crystal structure is rhombohedral.It is one of the few metals that expand onsolidification; the expansion is 3.3%. The thermalconductivity of bismuth is lower than thatof any metal, with the exception of mercury.Bismuth is the most diamagnetic of all metals(mass susceptibility of –1.35×106). It showsthe greatest Hall effect (increase in resistanceunder influence of a magnetic field). It also hasa low capture cross section for thermal neutrons(0.034 barn).Bismuth is playing an important role innuclear research. Its high density gives it excellentshielding properties for gamma rays whileits low thermal neutron capture cross sectionallows the neutrons to pass through. For investigationsin which it is desired to irradiateobjects, i.e., animals, with neutrons but protectthem from gamma rays, castings of bismuth areused as neutron windows in nuclear reactors.Bismuth has been proposed as a solventcoolantsystem for nuclear power reactors. Thebismuth dissolves sufficient uranium so that,when the solvent and solute are pumpedthrough a moderator (graphite), criticality isreached and fission takes place. The heat generatedfrom the fission reaction raises the temperatureof the bismuth. The heated bismuth isthen pumped to conventional heat exchangersproducing the steam power required for eventualconversion to electricity.
EXPOSURE ROUTES
inhalation, skin and/or eye contact
FIRST AID
(See procedures) Eye:Irrigate immediately Skin:Soap wash immediately Breathing:Respiratory support Swallow:Medical attention immediately
InChI:InChI=1/Bi
7440-69-9 Relevant articles
Interfacial reactions in Sn/Bi2Te3, Sn/Bi 2Se3 and Sn/Bi2(Te1-xSe x)3 couples
Chen, Sinn-Wen,Wu, Chih-Yu,Wu, Hsin-Jay,Chiu, Wan-Ting
, p. 313 - 318 (2014)
Bi2(Te1-xSex)3 is an important kind of n...
Syntheses and characterizations of bismuth nanofilms and nanorhombuses by the structure-controlling solventless method
Chen, Jing,Wu, Li-Ming,Chen, Ling
, p. 586 - 591 (2007)
Substrate-free bismuth nanofilms with an...
Facile synthesis of Bi/BiOCl composite with selective photocatalytic properties
Chen, Dongling,Zhang, Min,Lu, Qiuju,Chen, Junfang,Liu, Bitao,Wang, Zhaofeng
, p. 647 - 654 (2015)
This paper presents a novel and facile m...
Effect of the surface configuration on the oxidation of bismuth nanowire
Huang,Fung
, p. 1604 - 1611 (2006)
Incorporating nanoprocessing into the me...
Structure and resistivity of bismuth nanobelts in situ synthesized on silicon wafer through an ethanol-thermal method
Gao, Zheng,Qin, Haiming,Yan, Tao,Liu, Hong,Wang, Jiyang
, p. 3257 - 3261 (2011)
Bismuth nanobelts in situ grown on a sil...
Synthesis and single crystal X-ray structure analysis of bromodi(isopropenyl)bismuthane
Schumann, Herbert,Muehle, Stefan H.
, p. 629 - 632 (1999)
Tri(isopropenyl)bismuthane (1) reacts wi...
Structural and magneto-transport properties of electrodeposited bismuth nanowires
Liu, Kai,Chien,Searson,Yu-Zhang, Kui
, p. 1436 - 1438 (1998)
Arrays of semimetallic Bi nanowires have...
Bismuth oxychloride nanoflake assemblies as a new anode for potassium ion batteries
Li, Wei,Xu, Yang,Dong, Yulian,Wu, Yuhan,Zhang, Chenglin,Zhou, Min,Fu, Qun,Wu, Minghong,Lei, Yong
, p. 6507 - 6510 (2019)
This work reports the first demonstratio...
Orientation-controlled phase transformation of Bi2O3 during oxidation of electrodeposited Bi film
Huang, Chaur-Chi,Wen, Teng-Yi,Fung, Kuan-Zong
, p. 110 - 118 (2006)
High-temperature fluorite structure Bi2O...
The kinetics of thermal decomposition of bismuth oxohydroxolaurate
Logvinenko,Mikhailov,Yukhin
, p. 47 - 49 (2007)
The bismuth salt of lauric (dodecanic) a...
Potentiodynamic electrochemical impedance spectroscopy
Ragoisha,Bondarenko
, p. 1553 - 1563 (2005)
Potentiodynamic electrochemical impedanc...
A Room-Temperature Route to Bismuth Nanotube Arrays
Yang, Baojun,Li, Cun,Hu, Hanmei,Yang, Xiaogang,Li, Qiaowei,Qian, Yitai
, p. 3699 - 3702 (2003)
A room-temperature aqueous-chemical rout...
The Subbromide Bi5Br4 – On the Existence of a Hidden Phase
Pabst, Falk,Chang, Jen-Hui,Finzel, Kati,Kohout, Miroslav,Schmidt, Peer,Ruck, Michael
, p. 149 - 155 (2020)
Black and irregularly shaped crystals of...
The underpotential deposition of bismuth and tellurium on cold rolled silver substrate by ECALE
Zhu,Yang,Gao,Hou,Bao,Fan
, p. 5465 - 5472 (2005)
Thin-layer electrochemical studies of th...
Effect of Bi(III) concentration on the stripping voltammetric response of in situ bismuth-coated carbon paste and gold electrodes
Baldrianova,Svancara,Vlcek,Economou,Sotiropoulos
, p. 481 - 490 (2006)
The effect of Bi(III) concentration (ove...
Epitaxial Bi/GaAs(111) diodes via electrodeposition
Bao, Zhi Liang,Kavanagh, Karen L.
, p. 1 - 3 (2006)
Bismuth films formed by electrodepositio...
New single-source precursor for bismuth sulfide and its use as low-cost counter electrode material for dye-sensitized solar cells
Chauhan, Ratna,Chaturvedi, Jyotsna,Trivedi, Manoj,Singh, Jyoti,Molloy, Kieran C.,Kociok-K?hn, Gabriele,Amalnerkar, Dinesh P.,Kumar, Abhinav
, p. 168 - 175 (2015)
Abstract One new homoleptic [Bi(dtc)3] (...
Effects of polyethylene glycol and gelatin on the crystal size, morphology, and Sn2+-sensing ability of bismuth deposits
Tsai, Yi-Da,Lien, Chein-Hung,Hu, Chi-Chang
, p. 7615 - 7621 (2011)
The influences of citric acid (CA), ethy...
Electrodeposition of bismuth thin films on n-GaAs (110)
Vereecken, Philippe M.,Rodbell, Kenneth,Ji, Chunxin,Searson, Peter C.
, p. 1 - 3 (2005)
Bismuth thin films are formed electroche...
Bi electrodeposition under magnetic field
Motoyama,Fukunaka,Kikuchi
, p. 897 - 905 (2005)
Bi was galvanostatically electrodeposite...
Core-Shell Au?SnO2 Nanostructures Supported on Na2Ti4O9 Nanobelts as a highly active and deactivation-resistant catalyst toward selective nitroaromatics reduction
Pan, Xiaoyang,Zheng, Jing,Zhang, Liuxian,Yi, Zhiguo
, p. 11164 - 11171 (2019)
Catalysis using gold (Au) nanoparticles ...
Comparison of nucleophilic- and radical-based routes to the formation of manganese-main group element single bonds
Agnew, Douglas W.,Moore, Curtis E.,Rheingold, Arnold L.,Figueroa, Joshua S.
, p. 6700 - 6707 (2017)
Using the stable metalloradical Mn(CO)3(...
In situ STM study of underpotential deposition of bismuth on Au(1 1 0) in perchloric acid solution
Hara, Masanori,Nagahara, Yoshiki,Inukai, Junji,Yoshimoto, Soichiro,Itaya, Kingo
, p. 2327 - 2332 (2006)
The underpotential deposition (UPD) of B...
Volume plasmon of bismuth nanoparticles
Jiang, Nan,Su, Dong,Spence, John C.H.,Zhou, Shifeng,Qiu, Jianrong
, p. 111 - 114 (2009)
This paper reports the measurements of t...
Bismuth terephthalate induced Bi(0) for enhanced RhB photodegradation and 4-nitrophenol reduction
Zhao, Xinyun,Zhong, Jianping,Hu, Juncheng,Wu, Lamei,Chen, Xi
, p. 431 - 438 (2017)
A facile reductive method was used to pr...
Synthesis and characterization of bismuth single-crystalline nanowires and nanospheres
Wang, Junwei,Wang, Xun,Peng, Qing,Li, Yadong
, p. 7552 - 7556 (2004)
A facile solution-phase process has been...
A novel technique to extract Bi from mechanochemically prepared Bi-Fe 3O4 nanocomposite
Mozaffari,Amighian,Hasanpour
, p. 309 - 312 (2006)
The solid-state reduction of Bi2O3 to bi...
Pushing the Frontiers of Hard and Soft Scorpionate Chemistry
Dodds, Christopher A.,Kennedy, Alan R.,Reglinski, John,Spicer, Mark D.
, p. 394 - 395 (2004)
The preparation and structure of the fir...
Spin-Orbit Relaxation Rate of Bi(6p3, 2D3/2) following Photolysis of Bi(CH3)3 at λ = 193 nm
Holloway J. S.,Koffend, J. B.,Heidner, R. F. III
, p. 7665 - 7669 (1989)
Rate coefficients for the collisional re...
ELECTROLYTIC DEPOSITION OF BISMUTH ON CdS(0001) SINGLE-CRYSTAL SURFACES.
Elmorsi,Juettner
, p. 211 - 216 (1986)
The electrolytic deposition of Bi on CdS...
Reactions of MnO2, Mn2O3, α-Bi 2O3, and Bi12Ti1 - XMn xO20with supercritical isopropanol
Buslaeva,Kravchuk,Kargin,Gubin
, p. 582 - 585 (2002)
Heterogeneous reactions between supercri...
Bismuth spheres grown in self-nested cavities in a silicon wafer
Liu, Hong,Wang, Zhong Lin
, p. 15322 - 15326 (2005)
We have developed a one-step, hydrofluor...
Unveiling Intrinsic Potassium Storage Behaviors of Hierarchical Nano Bi@N-Doped Carbon Nanocages Framework via In Situ Characterizations
Sun, Zehang,Liu, Yang,Ye, Weibin,Zhang, Jinyang,Wang, Yuyan,Lin, Yue,Hou, Linrui,Wang, Ming-Sheng,Yuan, Changzhou
, p. 7180 - 7187 (2021)
Metallic bismuth has drawn attention as ...
Thermal properties of Bi nanowire arrays with different orientations and diameters
Zhu, Yonggang,Dou, Xincun,Huang, Xiaohu,Li, Ang,Li, Guanghai
, p. 26189 - 26193 (2006)
The thermal properties of single-crystal...
Synthesis and molecular structure of the novel bismuth(III) sulfonate complex [Bi(C18H14P(O)SO3)2 (DMSO) 3](NO3)·DMSO·2H2O
Schlesinger, Maik,Rueffer, Tobias,Lang, Heinrich,Mehring, Michael
, p. 135 - 139 (2012)
The synthesis of [Bi(C18H14P(O)SO3) 2(DM...
Synthesis of bis(tricarbonylcyclopentadienylmolybdenum)-bismuth(III) chloride and its reaction with metals
Piskunov,Maslennikov,Spirina,Artemov,Malysheva
, p. 1054 - 1056 (2003)
Polynuclear organometallic compounds [Cp...
Femtosecond transition-state absorption spectroscopy of Bi atoms produced by photodissociation of gaseous Bi2 molecules
Glownia, J. H.,Misewich, J. A.,Sorokin, P. P.
, p. 3335 - 3339 (1990)
Femtosecond transition-state absorption ...
N-Doped carbon encapsulating Bi nanoparticles derived from metal-organic frameworks for high-performance sodium-ion batteries
Chen, Huimin,Chen, Lin,He, Xiaojie,Huang, Shuping,Wei, Mingdeng
, p. 22048 - 22055 (2021/10/14)
Bismuth (Bi), as an alloy-based material...
Structural Optimization of Metal Oxyhalide for CO2 Reduction with High Selectivity and Current Density
Duan, Yan-Xin,Liu, Kai-Hua,Meng, Fan-Lu,Zhang, Qi,Zhang, Xin-Bo
supporting information, p. 1752 - 1756 (2020/11/27)
Electrochemical CO2 reduction into value...
7440-69-9 Process route
-
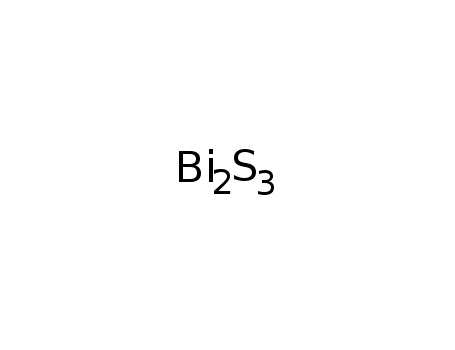
-
bismuth(III) sulfide

-

- 7440-69-9
bismuth

-
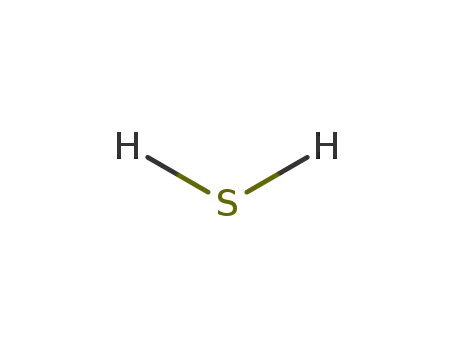
- 7783-06-4
hydrogen sulfide
ConditionsConditions Yield With hydrogen; reaction of Bi2S3 in a strem of H2 (2 l/hour), stimulating voltage 15 kV;;With hydrogen; In melt; melting Bi2S3 under H2 at 550-750°C;; Kinetics;With hydrogen; In neat (no solvent); heating at 400-600°C;;With hydrogen; In neat (no solvent); heating at 294-366°C;; Kinetics;With hydrogen; In neat (no solvent); heating at 650-750°C under N2;; Kinetics;With hydrogen; In neat (no solvent); heating of solid Bi2S3 under H2 at 294-366°C;; dynamic method of determination;; Kinetics;With hydrogen; In neat (no solvent); heating of solid Bi2S3 under H2 at 327-595°C;; statistic determination;; Kinetics;With hydrogen; In neat (no solvent); heating of solid Bi2S3 under H2 at 400-600°C;; dynamic and statistic determination;; Kinetics;With hydrogen; In neat (no solvent); heating of solid Bi2S3 under H2 at 414-765°C;; dynamic and statistic determination;; Kinetics;With hydrogen; In neat (no solvent); heating of solid Bi2S3 under H2 at 456-656°C;; statistic determination;; Kinetics;With mercury; zinc; In sulfuric acid; shaking of Bi2S3 with Zn amalgam under diluted aq. H2SO4; decomposition with formation of Bi and H2S;;With H2; In neat (no solvent); heating of solid Bi2S3 under H2 at 456-656°C;; statistic determination;; Kinetics;With H2; In neat (no solvent); heating of solid Bi2S3 under H2 at 414-765°C;; dynamic and statistic determination;; Kinetics;With H2; In melt; melting Bi2S3 under H2 at 550-750°C;; Kinetics;With H2; In neat (no solvent); heating Bi2S3 under H2S in presence of CaS (19.63 mol), SrS (20.05) or BaS (21.03) at 456-706°C; formation of Bi and H2S; decreasing of H2S/H2 ratio by the earth alkali sulfides;;With H2; In neat (no solvent); heating at 400-600°C;;With H2; In neat (no solvent); heating at 294-366°C;; Kinetics;With H2; In neat (no solvent); heating at 650-750°C under N2;; Kinetics;-
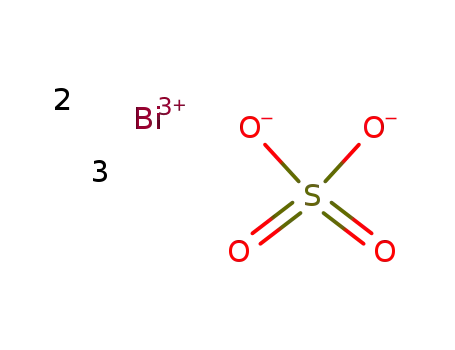
-
bismuth(III) sulfate

-

- 7440-69-9
bismuth

-
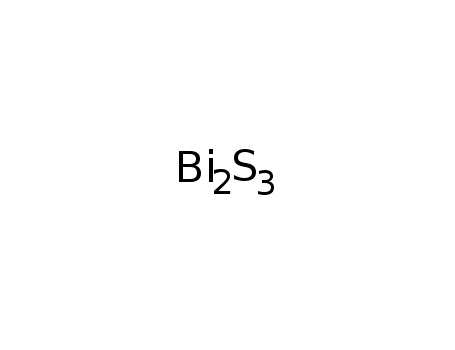
-
bismuth(III) sulfide

-

- 7783-06-4
hydrogen sulfide
ConditionsConditions Yield With hydrogen; In neat (no solvent); byproducts: H2O; reduction of Bi sulfate in a discharge tube at 15 kV under H2 (2 l/hour) at 25°C;;With H2; In neat (no solvent); byproducts: H2O; reduction of Bi sulfate in a discharge tube at 15 kV under H2 (2 l/hour) at 25°C;;With H2;7440-69-9 Upstream products
-
1304-76-3
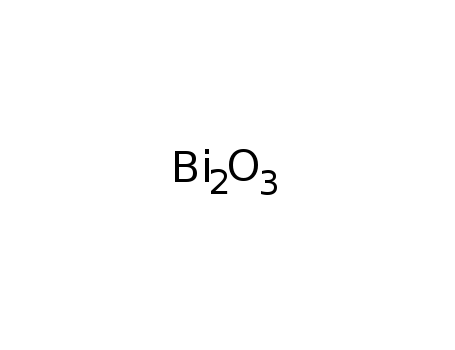
bismuth(III) oxide
-
64-18-6
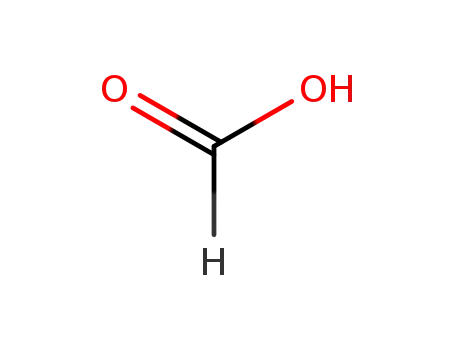
formic acid
-
603-33-8

triphenylbismuthane
-
10026-06-9
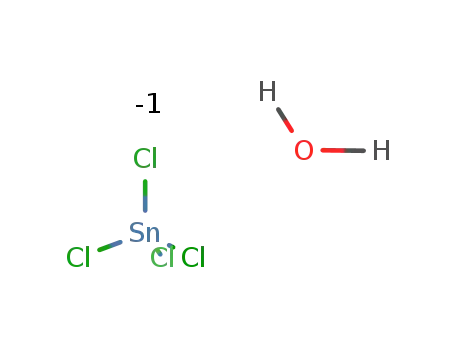
tin tetrachloride hydrate
7440-69-9 Downstream products
-
68282-49-5
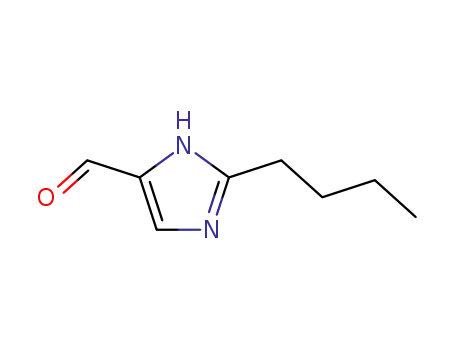
2-butyl-1H-imidazole-5-carboxaldehyde
-
4231-35-0
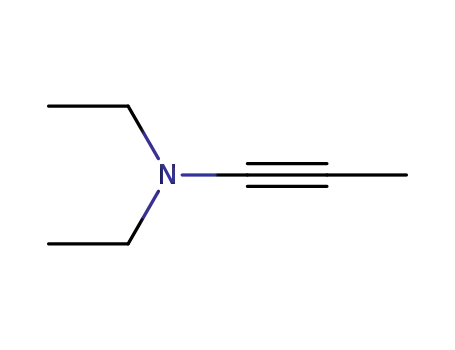
methyl(diethylamino)acetylene
-
7787-64-6
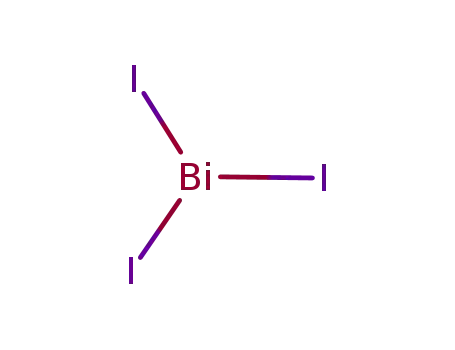
bismuth(III) iodide
-
13517-10-7
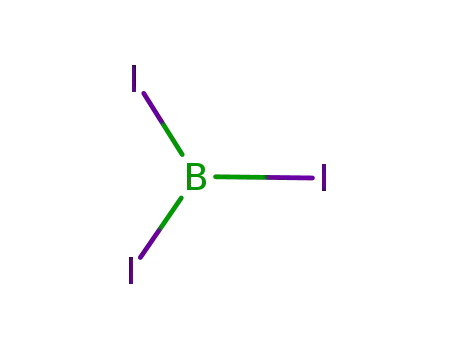
boron triiodide

