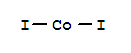- Molecular Formula: CoI2
- CasNo.: 15238-00-3
- Melting point: >515 °C(lit.)<BR>
- Appearance:
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
99.99% Pure Chinese Factory Supply 99.99% Chromium Iodide 15238-00-3 with Cheapest Price
- Molecular Formula:CoI2
- Molecular Weight: 312.74
- Melting Point:>515 °C(lit.)
- Boiling Point:570°C
- Flash Point:570oC vac.
- PSA:0.00000
- Density:5.68
- LogP:1.77140
COBALT(II) IODIDE(Cas 15238-00-3) Usage
Preparation
Cobalt(II) iodide is prepared by heating cobalt powder in a stream of hydrogen iodide at 400 to 450°C: Co + 2HI → CoI2 + H2 The product obtained is the black crystalline α-form. Cobalt(II) iodide also may be made by heating cobalt powder with iodine vapor.
Physical properties
Exists in two isomorphous forms, α- and ?-forms; both modifications highly hygroscopic. The α-form is black hexagonal crystal; density 5.58 g/cm3; turns dark green in air; melts at 560°C; disolves in water giving pink coloration. The α-forms sublimes in vacuo, partly forming an isomorous yellow modification-the anhydrous β-form. The β-modification is a yellow powder; density 5.45 g/cm3; converts to the α-form when heated to 400°C; absorbs moisture from air, the yellow powder becoming green droplets; dissolves readily in water forming a colorless solution which turns pink on heating. The hexahydrate is red hexagonal crystals; density 2.90 g/cm3; loses water at 130°C giving anhydrous iodide; soluble in water, ethanol, acetone, chloroform and ether, forming colored solutions, (while the aqueous solution is red below 20°C and green above this temperature; the salt forms blue solution in ethanol, chloroform and ether).
InChI:InChI=1/Co.2HI/h;2*1H/q+2;;/p-2