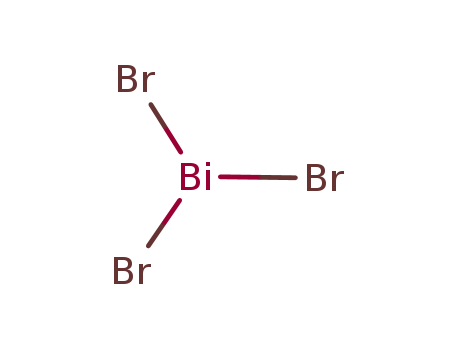
- Molecular Formula: BiBr3
- CasNo.: 7787-58-8
- Melting point: 218 °C
- Appearance: Light yellow-gray crystals or crystalline powder
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
Best Quality Reputable Factory Supply 99.99% Bismuth Bromide 7787-58-8 with Cheap Price
- Molecular Formula:BiBr3
- Molecular Weight:448.692
- Appearance/Colour:Light yellow-gray crystals or crystalline powder
- Melting Point:218 °C
- Boiling Point:453 °C
- Flash Point:453°C
- PSA:0.00000
- Density:5.7 g/mL at 25 °C(lit.)
- LogP:2.53680
Bismuth(III) bromide(Cas 7787-58-8) Usage
General Description
Bismuth(III) bromide, also known as bismuth tribromide, is an inorganic compound with the formula BiBr3. It is a heavy, crystalline solid that appears as a dark reddish-brown color, but in its pure form, it is colorless. This substance has a molecular weight of 558.52 g/mol and a melting point of 218 °C. It is a strong Lewis acid and it is regarded as being corrosive. Bismuth(III) bromide can conduct electricity due to the presence of mobile ions and it is highly soluble in water, alcohol, and acetone. It's often used as a reagent in organic synthesis. However, it is extremely sensitive and may react violently when it comes into contact with other chemicals, thus proper handling and storage is needed.
InChI:InChI=1/Bi.3BrH/h;3*1H/q+3;;;/p-3
7787-58-8 Relevant articles
Metal-organic supramolecular assemblies generated from bismuth(III) bromide and polyimine ligands
Soltanzadeh, Nilofar,Morsali, Ali
, p. 703 - 710 (2009)
Three new BiBr3 supramolecular complexes...
Electronic structure, galvanomagnetic and magnetic properties of the bismuth subhalides Bi4I4 and Bi4Br4
Filatova,Gurin,Kloo,Kulbachinskii,Kuznetsov,Kytin,Lindsjo,Popovkin
, p. 1103 - 1109 (2007)
Two bismuth-rich subhalides, Bi4Br4 and ...
Synthesis and structures of novel ring compounds of bismuth with tris(trimethylsilyl)silyl and -stannyl substituents - [(Me3Si)3Si]4Bi4 and [(Me3Si)3Sn]6Bi8
Linti, Gerald,Koestler, Wolfgang
, p. 63 - 66 (2002)
A bicyclo[3.3.0]octane-like core consist...
Syntheses, crystal structures, and triple twinning of the cluster trimers Bi2[PtBi6Br12]3 and Bi 2[PtBi6I12]3
Guenther, Anja,Steden, Folker,Ruck, Michael
, p. 423 - 430 (2008)
Melting reactions of Bi with Pt and BiX3...
Structure and bonding in chloro- and bromobismuthate(III) clusters (BiX4-, Bi2X93-, BiX63-) by NQR spectroscopy
Landers,Brill
, p. 744 - 749 (1980)
The structural aspects of difficultly ch...
Homologous silver bismuth chalcogenide halides (N, x)P. I. syntheses and crystal structures of the (0, 1)P compound AgBi 2S2Cl3 and of three members of the (1, x)P solid solution series Ag2xBi4-2xS 6-4xBr4x
Poudeu, Pierre Ferdinand Poudeu,Soehnel, Tilo,Ruck, Michael
, p. 1276 - 1285 (2004)
AgBi2S2Cl3 and three members of the Ag2x...
N-Heterocyclic carbene adducts of the heavier group 15 tribromides. Normal to abnormal isomerism and bromide ion abstraction
Waters, Jordan B.,Chen, Qien,Everitt, Thomas A.,Goicoechea, Jose M.
, p. 12053 - 12066 (2017/09/25)
The reactivity of the heavier group 15 t...
Trapping molecular bromine: A one-dimensional bromobismuthate complex with Br2 as a linker
Adonin,Gorokh,Abramov,Plyusnin,Sokolov,Fedin
, p. 3691 - 3693 (2016/03/05)
The reaction between solid (NMP)n{[BiBr4...
7787-58-8 Process route
-
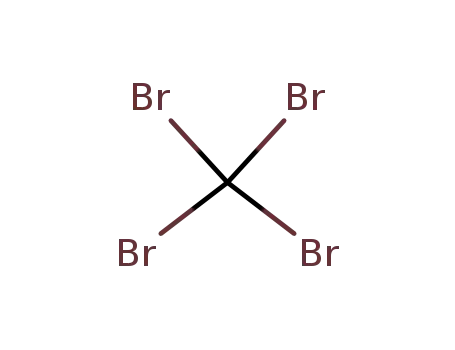
- 558-13-4
carbon tetrabromide

-
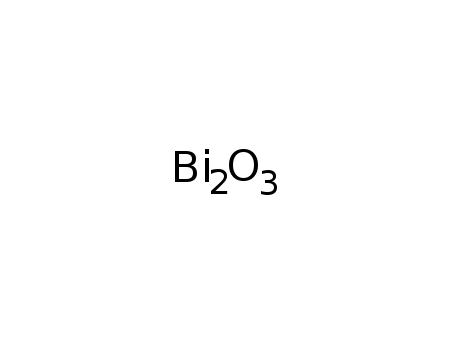
- 1304-76-3
bismuth(III) oxide

-
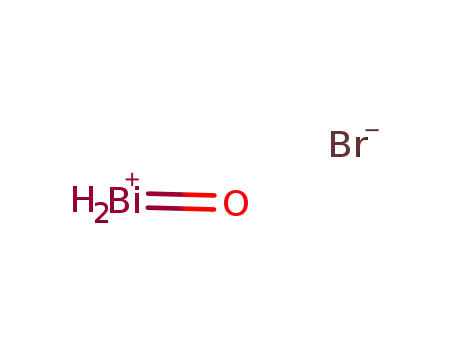
- 7787-57-7
bismuth oxobromide

-
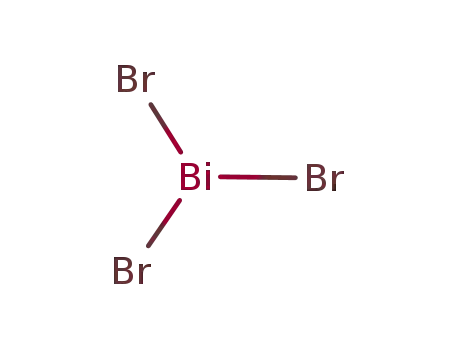
- 7787-58-8
bismuth(III) bromide
ConditionsConditions Yield In neat (no solvent); byproducts: CO2, CO; heating Bi2O3 and CBr4 in vacuum at 200°C; various yields for various educt ratio;;19.9-90.6
9.4-80.1In neat (no solvent); byproducts: CO2, CO; heating Bi2O3 and CBr4 in vacuum at 200°C; various yields for various educt ratio;;19.9-90.6
9.4-80.1-
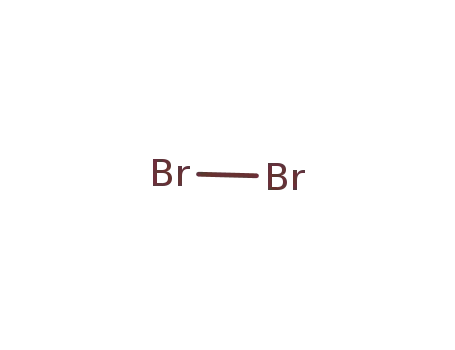
- 7726-95-6
bromine

-

- 1304-76-3
bismuth(III) oxide

-
- 7787-57-7
bismuth oxobromide

-

- 7787-58-8
bismuth(III) bromide
ConditionsConditions Yield formation of a mixture of BiBr3 and BiOBr;;formation of a mixture of BiBr3 and BiOBr;;7787-58-8 Upstream products
-
7726-95-6
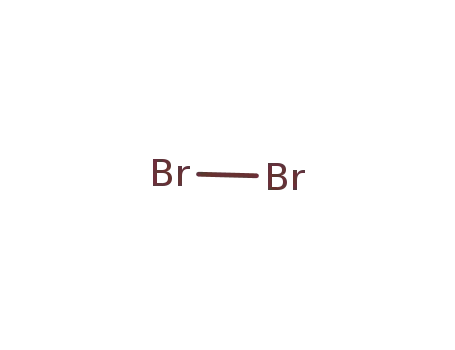
bromine
-
1304-76-3

bismuth(III) oxide
-
558-13-4

carbon tetrabromide
-
46140-16-3
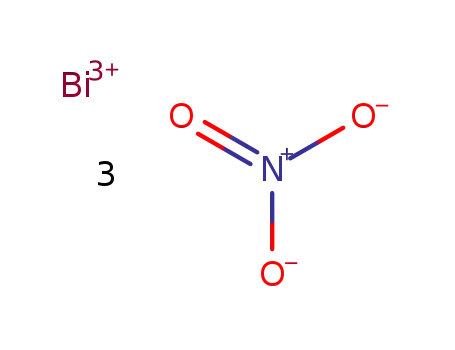
bismuth(III) nitrate
7787-58-8 Downstream products
-
871810-89-8
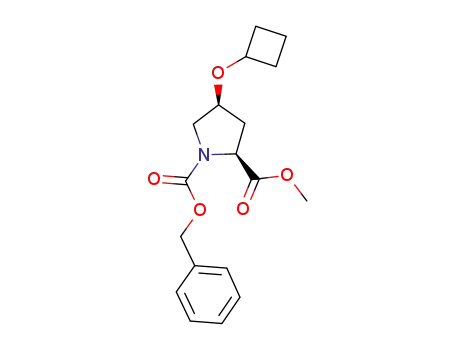
(1-benzyloxycarbonyl-(4S)-cyclobutoxy-(2S)-pyrrolidinyl)carboxylic acid methyl ester
-
7440-69-9

bismuth
-
603-33-8

triphenylbismuthane
-
39110-02-6
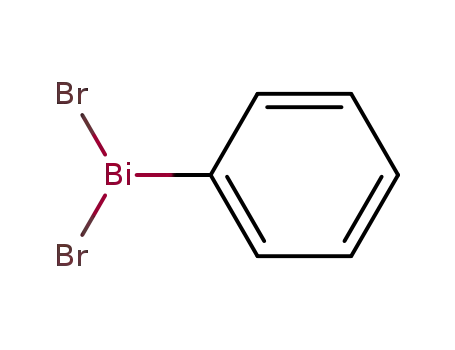
dibromophenylbismuthane