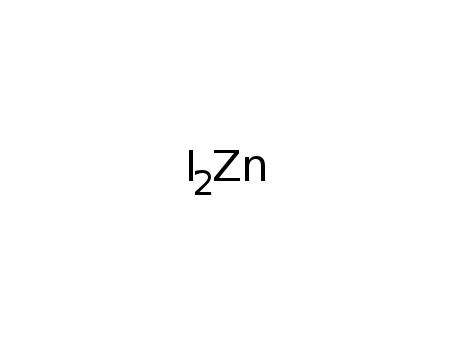
- Molecular Formula: ZnI2
- CasNo.: 10139-47-6
- Melting point: 445 °C(lit.)
- Appearance: White crystalline powder
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
High Purity 99% Quality Factory Supply 99.99%-99.999% Zinc Iodide 10139-47-6 with Cheap Price
- Molecular Formula:I2Zn
- Molecular Weight:319.199
- Appearance/Colour:White crystalline powder
- Melting Point:445 °C(lit.)
- Boiling Point:624 °C
- Flash Point:625°C
- PSA:0.00000
- Density:4.74 g/mL at 25 °C(lit.)
- LogP:1.76890
Zinc iodide(Cas 10139-47-6) Usage
General Description
Zinc iodide is a chemical compound with the formula ZnI2, consisting of one zinc atom and two iodine atoms. It is a white, crystalline solid with a high melting point and is highly soluble in water. Zinc iodide is commonly used in organic synthesis as a Lewis acid catalyst, particularly in the formation of carbon-carbon and carbon-nitrogen bonds. It is also used in the production of ointments and dental cements, as well as in nuclear energy applications. Additionally, it has potential applications in the production of LEDs and as a stabilizer in PVC manufacturing. Overall, zinc iodide is a versatile and important chemical compound with a wide range of industrial and scientific applications.
InChI:InChI=1/2HI.Zn/h2*1H;/q;;+2/p-2
10139-47-6 Relevant articles
Preparation of stable and metastable coordination compounds: Insight into the structural, thermodynamic, and kinetic aspects of the formation of coordination polymers
Naether, Christian,Bhosekar, Gaurav,Jess, Inke
, p. 8079 - 8087 (2007)
The reaction of Znl2 and pyrimidine in a...
New framework lodoargentates: M(en)3Ag2I4 (M = Zn, Ni) with tridymite topology
Jiang, Yan-Si,Yao, Hua-Gang,Ji, Shou-Hua,Ji, Min,An, Yong-Lin
, p. 3922 - 3924 (2008)
Two novel framework compounds, Zn(en)3Ag...
-
Parsons, L. B.
, p. 1830 - 1835 (1925)
-
-
Blachnik, R.,Stoeter, U.
, p. 115 - 122 (1989)
-
Cyclotrimerization of terminal alkynes catalyzed by the system of NiCl 2/Zn and (benzimidazolyl)-6-(1-(arylimino)ethyl)pyridines
Xi, Chanjuan,Sun, Zelin,Liu, Yongbing
, p. 13327 - 13330 (2013)
An effective regioselective cyclotrimeri...
Synthesis and molecular structure of two zinc complexes of 1,2-bis[(trimethylsilyl)imino]acenaphthene
Fedushkin, Igor L.,Skatova, Alexandra A.,Eremenko, Olga V.,Hummert, Markus,Schumann, Herbert
, p. 1739 - 1742 (2007)
The reaction of 1,2-bis[(trimethylsilyl)...
Synthesis, crystal structures, and thermal properties of new [ZnX 2(2,5-dimethylpyrazine)] (X = Cl, Br, I) coordination compounds
Wriedt, Mario,Jess, Inke,Naether, Christian
, p. 363 - 372 (2009)
Eight new halidozinc(II) coordination co...
Revealing the structural chemistry of the group 12 halide coordination compounds with 2,2′-bipyridine and 1,10-phenanthroline
Swiatkowski, Marcin,Kruszynski, Rafal
, p. 642 - 675 (2017/02/05)
The coordination compounds of group 12 h...
Stereoselective Retentive Domino Transmetalations of Secondary Alkyllithium Compounds to Functionalized Secondary Alkylcopper Reagents
Moriya, Kohei,Simon, Meike,Mose, Rasmus,Karaghiosoff, Konstantin,Knochel, Paul
supporting information, p. 10963 - 10967 (2015/09/15)
Functionalized secondary alkyllithium re...
STABILIZED COMPOSITIONS AND METHODS OF MANUFACTURE
-
Paragraph 0026, (2014/09/30)
A method for stabilization of potent alk...
Novel inhibitors of bacterial virulence: Development of 5,6-dihydrobenzo[h]quinazolin-4(3H)-ones for the inhibition of group A streptococcal streptokinase expression
Yestrepsky, Bryan D.,Xu, Yuanxi,Breen, Meghan E.,Li, Xiaoqin,Rajeswaran, Walajapet G.,Ryu, Jenny G.,Sorenson, Roderick J.,Tsume, Yasuhiro,Wilson, Michael W.,Zhang, Wenpeng,Sun, Duxin,Sun, Hongmin,Larsen, Scott D.
, p. 1880 - 1897 (2013/05/08)
Resistance to antibiotics is an increasi...
10139-47-6 Process route
-
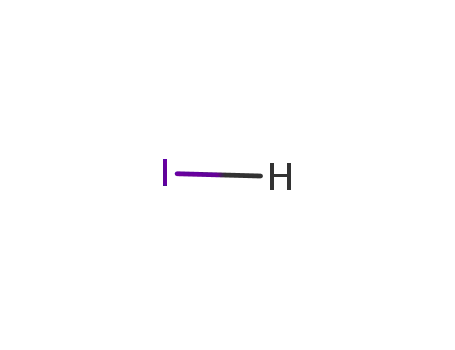
- 10034-85-2
hydrogen iodide

-
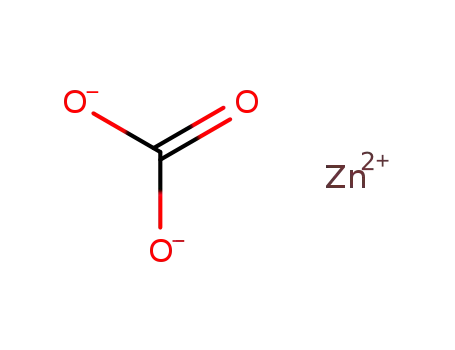
- 743369-26-8
zinc(II) carbonate

-
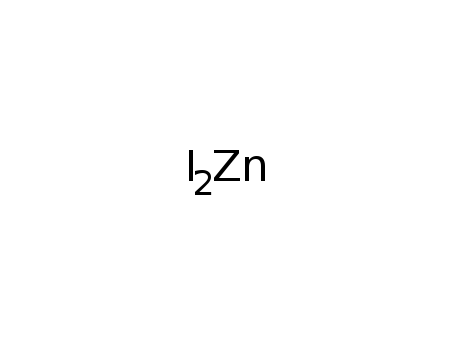
- 10139-47-6
zinc(II) iodide
ConditionsConditions Yield In further solvent(s); dissolved in HI;;With phosphorus; In water; Inert atmosphere;-
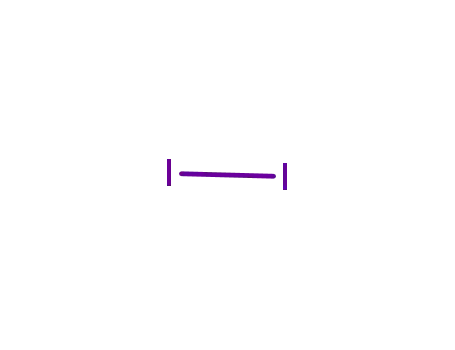
- 7553-56-2,12190-71-5,8031-47-8
iodine

-

- 7440-66-6
zinc

-

- 10139-47-6
zinc(II) iodide
ConditionsConditions Yield In N,N-dimethyl acetamide;reaction of Zn-sheet with iodine;;With iodine; In water; diluting pure electrolytic Zn in iodine solution;; Kinetics;In ethylbenzene;0% In pyridine;In tetrachloromethane;0% In ethanol;In water; Electrochem. Process; iodine accumulator; electrodes: graphite coal, Zn (vessel); soln. of electrolytes: aq. ZnI2 in porous special mass; cellulose diaphragm;;In water; digesting of Zn with I2 and H2O at 30-40°C, evaporation on slight warming;;In neat (no solvent); reaction starts already at moderate warmth with finely powdered educts;;In diethyl ether;In n-heptane;0% In carbon disulfide;0% In acetone;In quinoline;In benzene;0% In chloroform;0% In neat (no solvent); stoich. amts. sealed and melted in an evacuated silica ampoule;With iodine solution; In water; diluting pure electrolytic Zn in iodine solution;; Kinetics;reaction of Zn-sheet with iodine;;In water; digesting of Zn with I2 and H2O at 30-40°C, evaporation on slight warming;;In water; Electrochem. Process; iodine accumulator; electrodes: graphite coal, Zn (vessel); soln. of electrolytes: aq. ZnI2 in porous special mass; cellulose diaphragm;;In neat (no solvent); on react. of iodine vapor with liq. uinc at 600°C in a stream of N2;;In not given; excess Zn metal reacted with iodine;In diethylene glycol dimethyl ether; Inert atmosphere; Heating;In diethyl ether;In diethyl ether; for 72h;zinc; for 0.0833333h; Inert atmosphere; Schlenk technique; Heating;iodine; In diethyl ether; at 0 ℃; Inert atmosphere; Schlenk technique;10139-47-6 Upstream products
-
7553-56-2

iodine
-
7440-66-6

zinc
-
10034-85-2

hydrogen iodide
-
743369-26-8

zinc(II) carbonate
10139-47-6 Downstream products
-
14900-04-0
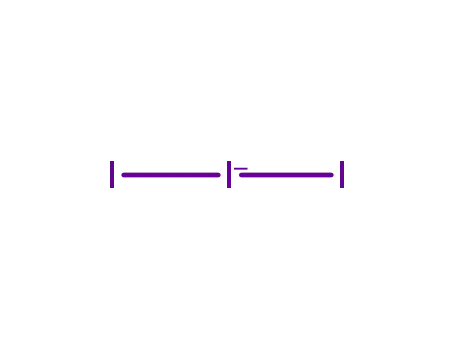
triiodide(1-)
-
14876-64-3
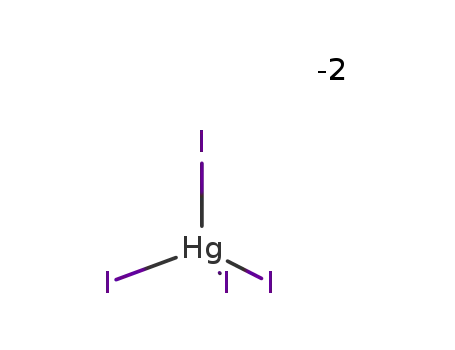
tetraiodomercurate(II)(2-)
-
105223-45-8
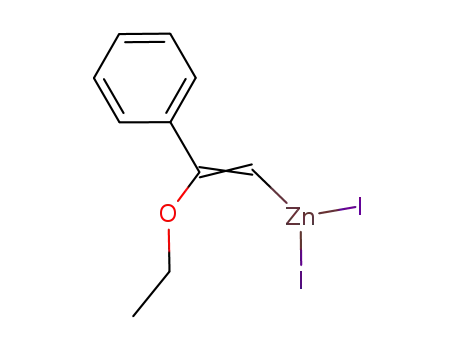
C6H5C(OC2H5)CHZnI2
-
14430-80-9
I2(p-toluidine)2zinc(II)