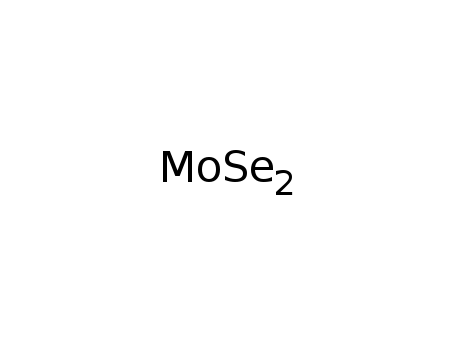
- Molecular Formula: MoSe2
- CasNo.: 12058-18-3
- Melting point: >1200 °C
- Appearance: crystalline solid
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
Reliable Quality Quality Manufacturer Supply 99.9%-99.99% Molybdenum(IV) Selenide 12058-18-3 On Stock
- Molecular Formula:MoSe2
- Molecular Weight:253.86
- Appearance/Colour:crystalline solid
- Melting Point:>1200 °C
- PSA:0.00000
- Density:6 g/cm3
- LogP:-0.53660
MOLYBDENUM SELENIDE(Cas 12058-18-3) Usage
Synthesis
High-quality molybdenum diselenide monolayer?film?was grown directly on the substrates (SiO2/Si) by the chemical vapour deposition (CVD) method.
Application
Having a?direct optical band gap of 1.48 eV with a photoluminescence peak at 840 nm, molybdenum diselenide monolayer?film?is?ideal for applications in optoelectronics. Also, with?with its narrower bandgap, higher optical absorbance and larger spin-splitting energy than MoS2,?MoSe2?ultrathin films?are potentially?better than MoS2 for the applications in tunnel FETs and optoelectronic devices.
Definition
Available as a 40-micron powder.
InChI:InChI=1/Mo.2Se/rMoSe2/c2-1-3
12058-18-3 Relevant articles
Synthesis of MoSe2 nanocrystallites by a solvothermal conversion from MoO3
Zhan,Zhang,Qian,Wang,Xie,Qian
, p. 497 - 501 (1999)
Nanocrystalline 2H-MoSe2 was prepared fr...
MoSe2/CdSe Heterojunction Destruction by Cation Exchange for Photoelectrochemical Immunoassays with a Controlled-Release Strategy
Leng, Dongquan,Zhao, Jihao,Ren, Xiang,Xu, Rui,Liu, Lei,Liu, Xuejing,Li, Yuyang,Wei, Qin
, p. 10712 - 10718 (2021)
Herein, a split-type immunoassay strateg...
Controllable synthesis of flower-like MoSe2 3D microspheres for highly efficient visible-light photocatalytic degradation of nitro-aromatic explosives
Huang, Jingwen,Jin, Bo,Liu, Huiqiang,Li, Xiaojuan,Zhang, Qingchun,Chu, Shijin,Peng, Rufang,Chu, Sheng
, p. 11424 - 11434 (2018)
Nitro-aromatic explosives existing on th...
Structural, optical and microscopic studies of tungsten substituted molybdenum diselenide thin films
Sathe,Hankare,Manikshete,Chate,Patil
, p. 187 - 193 (2010)
A modified chemical bath deposition meth...
Cr1.45Tl1.87Mo15Se19, a monoclinic variant of the hexa-gonal In3Mo15Se 19 type
Gougeon,Salloum,Potel
, p. i87-i90 (2009)
The monoclinic compound Cr1.45Tl1.87Mo 1...
Kinetics and Diffusing Species in the Reaction between Molybdenum and Selenium Vapor
Sasaki, Yoshinori,Wakatsuki, Noboru
, p. 863 - 864 (1993)
Metallic molybdenum was selenidized at t...
Modulating in-plane electron density of molybdenum diselenide via spontaneously atomic-scale palladium doping enables high performance lithium oxygen batteries
He, Miao,Hu, Anjun,Li, Jiabao,Li, Minglu,Long, Jianping,Shu, Chaozhu,Yan, Yu
, (2021)
Lithium-oxygen batteries (LOBs) are cons...
Preparation of MoSe2 nano-islands array embedded in a TiO2 matrix for photo-regulated resistive switching memory
Han, Pengde,Sun, Bai,Cheng, Sen,Yu, Fangli,Jiao, Baoxiang,Wu, Qisheng
, p. 619 - 625 (2016)
The electrically driven resistance chang...
Synthesis and characterization of indium intercalation compounds of molybdenum sulphoselenide
Mandal,Srivastava
, p. 3191 - 3196 (1996)
The synthesis, structure and properties ...
Dual surfactants applied in synthesis of MoSe2 for high-efficiency hydrogen evolution reaction
Li, Changdian,Zhu, Lili,Li, Han,Li, Hui,Wu, Ziqiang,Liang, Changhao,Zhu, Xuebin,Sun, Yuping
, (2021)
Molybdenum diselenide (MoSe2) has been c...
Cu Insertion into the Mo12 Cluster Compound Cs2Mo12Se14: Synthesis, Crystal and Electronic Structures, and Physical Properties
Al Rahal Al Orabi, Rabih,Fontaine, Bruno,Gautier, Regis,Gougeon, Patrick,Gall, Philippe,Bouyrie, Yohan,Dauscher, Anne,Candolfi, Christophe,Lenoir, Bertrand
, p. 6616 - 6624 (2016)
Mo-based cluster compounds are promising...
Hierarchical Co0.85Se-CdSe/MoSe2/CdSe Sandwich-Like Heterostructured Cages for Efficient Photocatalytic CO2 Reduction
Du, Lizhi,Chen, Yajie,Wang, Qi,Zhao, Yumeng,Li, Longge,Liu, Xiu,Tian, Guohui
, (2021)
Fabricating efficient photocatalysts wit...
Formation and interlayer decoupling of colloidal MoSe2 nanoflowers
Sun, Du,Feng, Simin,Terrones, Mauricio,Schaak, Raymond E.
, p. 3167 - 3175 (2015)
We report the colloidal synthesis of sub...
Superconducting properties of solid solutions (Mo6Se8)Pbx and PbxMo6Se8 in the ternary system Pb-Mo-Se
Corrignan, Anita,Hamard, Christelle,Pena, Octavio
, p. 260 - 264 (1999)
The homogeneity regions of the supercond...
Syntheses and Structural, Physical, and Theoretical Studies of the Novel Isostructural Mo9 Cluster Compounds Ag2.6CsMo 9Se11, Ag4.1ClMo9Se11, and h-Mo9Se11 with Tunnel Structures
Gougeon, Patrick,Potel, Michel,Gautier, Regis
, p. 1257 - 1263 (2004)
The new isostructural compounds Ag2.6CsM...
Synergetic effect of TiO2 as co-catalyst for enhanced visible light photocatalytic reduction of Cr(VI) on MoSe2
Chu, Haipeng,Lei, Wenyan,Liu, Xinjuan,Li, Jinliang,Zheng, Wei,Zhu, Guang,Li, Can,Pan, Likun,Sun, Changqing
, p. 19 - 25 (2016)
MoSe2-TiO2 composites were successfully ...
Influence of S and Te substitutions on the thermoelectric properties of the cluster compound Ag3.8Mo9Se11
Masschelein,Candolfi,Dauscher,Gendarme,Rabih, Al Rahal Al Orabi,Gougeon,Potel,Gall,Gautier,Lenoir
, p. 360 - 367 (2018)
We report on a detailed study of the inf...
Graphene-like 2H/1T-MoSe2 with superior full spectrum absorption: Morphology and phase engineering
Wu, Jinzhu,Liu, Yue,Yao, Yuan,Shao, Yanbin,Wu, Xiaohong
, (2021)
Superabsorbers can find application in t...
Phase Transition and Superconductivity Enhancement in Se-Substituted MoTe2 Thin Films
Li, Peiling,Cui, Jian,Zhou, Jiadong,Guo, Dong,Zhao, Zhenzheng,Yi, Jian,Fan, Jie,Ji, Zhongqing,Jing, Xiunian,Qu, Fanming,Yang, Changli,Lu, Li,Lin, Junhao,Liu, Zheng,Liu, Guangtong
, (2019)
Consecutively tailoring few-layer transi...
Non-stoichiometry and structure of molybdenum diselenide
Naruke,Wakatsuki,Hoshi,Sasaki
, p. 647 - 655 (1996)
Molybdenum diselenides were synthesized ...
Photoelectrochemical reduction of N2to NH3under ambient conditions through hierarchical MoSe2@g-C3N4heterojunctions
Mushtaq, Muhammad Asim,Arif, Muhammad,Fang, Xiaoyu,Yasin, Ghulam,Ye, Wen,Basharat, Majid,Zhou, Bo,Yang, Shiyu,Ji, Shengfu,Yan, Dongpeng
, p. 2742 - 2753 (2021)
Ammonia is the main precursor for the pr...
Chevrel-phase solid solution Mo6Se8-xTex. Study of its superconducting, magnetic and NMR properties
Hamard,Auffret,Pena,Le Floch,Nowak,Wojakowski
, p. 339 - 349 (2000)
The Chevrel-phase solid solution Mo6Se8-...
Synthesis, Crystal Structure, and Transport Properties of the Hexagonal Mo9 Cluster Compound Ag3RbMo9Se11
Gougeon, Patrick,Gall, Philippe,Merdrignac-Conanec, Odile,Aranda, Lionel,Dauscher, Anne,Candolfi, Christophe,Lenoir, Bertrand
, p. 9684 - 9692 (2017)
Mo-based cluster compounds are promising...
Single-Crystal Studies of the Chevrel-Phase Superconductor LaxMo6Se8: I: Correlation betweenTcand the Interatomic Distances
Le Berre,Pena,Perrin,Sergent,Horyn,Wojakowski
, p. 151 - 159 (1998)
We report, for the first time in the lit...
Two-dimensional graphene-like MoSe2 nanosheets anchored on hollow carbon nanofibers as a cathode catalyst for rechargeable Li-O2 batteries
Lai, Yanqing,Chen, Wei,Zhang, Zhian,Gan, Yongqing,Yang, Xing,Li, Jie
, p. 19843 - 19847 (2016)
MoSe2@HCNF hybrids are synthesized via a...
A novel route to obtain molybdenum dichalcogenides by hydrothermal reaction
Fan, Rong,Chen, Xianhui,Chen, Zuyao
, p. 920 - 921 (2000)
Hydrothermal reactions between aqueous N...
Ag2.54Tl2Mo12Se15: A new structure type containing Mo6 and Mo9 clusters
Gougeon,Gall,Gautier,Potel
, p. i67-i70 (2010)
The novel structure-type Ag2.54Tl2Mo 12S...
Crystal data for mixed-anion molybdenum dichalcogenides
Schneemeyer, Lynn F.,Sienko
, p. 789 - 791 (1980)
-
CoSe2/MoSe2 Heterostructures with Enriched Water Adsorption/Dissociation Sites towards Enhanced Alkaline Hydrogen Evolution Reaction
Zhao, Guoqiang,Li, Peng,Rui, Kun,Chen, Yaping,Dou, Shi Xue,Sun, Wenping
, p. 11158 - 11165 (2018)
Transition-metal dichalcogenides (TMDs) ...
Optimization of MoSe2 nanostructure by surface modification using conducting polymer for degradation of cationic and anionic dye: Photocatalysis mechanism, reaction kinetics and intermediate product study
Mittal, Honey,Khanuja, Manika
, (2020)
In the present work, optimum nanocomposi...
Synthesis and characterization of indium intercalation compounds of molybdenum diselenide, InxMoSe2 (0 ≤ x ≤ 1)
Srivastava,Avasthi
, p. 1919 - 1924 (1989)
The synthesis, structure and properties ...
One-pot hydrothermal synthesis and selective etching method of a porous MoSe2 sand rose-like structure for electrocatalytic hydrogen evolution reaction
Tran, Xuan Thai,Poorahong, Sujittra,Siaj, Mohamed
, p. 52345 - 52351 (2017)
The development of a platinum-free elect...
In-situ transformation into MoSe2/MoO3 heterogeneous nanostructures with enhanced electrochemical performance as anode material for sodium ion battery
Kang, Wenpei,Wang, Yuyu,Cao, Dongwei,Kang, Zixi,Sun, Daofeng
, p. 410 - 418 (2018)
As anode materials, the electrochemical ...
X-ray characterization, electronic band structure, and thermoelectric properties of the cluster compound Ag2Tl2Mo9Se11
Al Rahal Al Orabi, Rabih,Gougeon, Patrick,Gall, Philippe,Fontaine, Bruno,Gautier, Rgis,Colin, Malika,Candolfi, Christophe,Dauscher, Anne,Hejtmanek, Jiri,Malaman, Bernard,Lenoir, Bertrand
, p. 11699 - 11709 (2014)
We report on a detailed investigation of...
1T-2H MoSe2 modified MAPbI3 for effective photocatalytic hydrogen evolution
Cai, Yifei,Chen, Jinxi,Lou, Yongbing,Zhang, Tiantian
, (2021/10/25)
Organic-inorganic perovskites such as io...
Microwave hydrothermal synthesis of hierarchical Ce-doped MoSe2@CNTs as an efficient non-precious catalyst for hydrogen evolution in both acidic and alkaline media
Chen, Zhidong,Ji, Dingwei,Liu, Changhai,Luo, Linlin,Wang, Wenchang,Yao, Yanhua
, (2021/11/16)
To enhance the intrinsic activity and th...
Synthesis, Structure, and Spectroscopic Study of Redox-Active Heterometallic Cluster-Based Complexes [Re5MoSe8(CN)6]n
Cordier, Stéphane,Dorcet, Vincent,Loginov, Ivan P.,Muravieva, Viktoria K.,Nadolinny, Vladimir A.,Naumov, Nikolay G.,Ryzhikov, Maxim R.,Sukhikh, Taisiya S.,Yanshole, Vadim V.
, p. 8838 - 8850 (2021/06/27)
The heterometallic cluster-based compoun...
12058-18-3 Process route
-

- 7782-49-2
selenium

-

- 7439-98-7
molybdenum

-
- 12058-18-3
molybdenum selenide
ConditionsConditions Yield at 800,900,1000 and 1150°C;Kinetics;In neat (no solvent); slow heating of weighed mixt. (sealed evacuated quartz ampoule, ca. 1E-5 Torr) to 950°C (horizontal furnace), maintaining there for 1 week; powder X-ray diffraction;sheet of molybdenum and an excess of selenium shots placed at ends of a silica tube, tube sealed under vac., selenidization at 773-873 K (selenium vapor at 1.33 kPa); Kinetics;In neat (no solvent); Mo and Se powder weighed accurately, placed in a quartz tube, vacuum sealed, initial react. at 750 ° C over 40 h,, resulted powder mechanical shaking, final react. at 1000 ° C over 40 h, slow cooling to 25 ° C;In neat (no solvent); heating (sealed quartz ampoule, 873 K); X-ray diffraction;In neat (no solvent); reacting Se vapor with powdered metal at 1000K in a flowing inert gas orby reacting the components in evacuated quartz ampules, utilizing a particle size of less than 50 μm; x-ray diffraction;at 800,900,1000 and 1150°C;In neat (no solvent); Mo powder and Se shot were mixed, sealed in a quartz ampoule, and heated at 600°C for 3 d, after remixing, the sample was homogenized by heating at 1000°C for further 4 d;;stoichiometric mixtures of elements were sealed in evacuated silica tubes; heated to 450°C for two days; fired at 1000°C for 10 days;With Te; In neat (no solvent); Mo and Te thin films sequentially deposited on a polished glass substrate, annealing under Se and Te pressure at 770 K, 24 h; crystallized stoich. film;In neat (no solvent); formation of MoSe2 in an evacuated quartz capsule;; elem. anal. given; X-ray diffraction;;In neat (no solvent, solid phase); stoich. proportion; evacuated; sealed (1E-5 torr); mixed; slowly heatedto 800°C (40°/h); after 72 h cooled; heated at 1040°C (168 h); ESCA;stoichiometric amounts of elements heated in a degassed, sealed, evacuated silica tube upto 900°C over a period of 5 days and kept at this temp. for 1 week; cooled to room temp. then reheated to 900°C for another week;In neat (no solvent); heating (dual-chamber quartz tube, full consumption of Se vapors);In neat (no solvent, solid phase); at 850°C for 3 d in quartz tube;mixt. grinding (agate mortar), heating in evac. sealed (under Ar) quartztube at 1273+/-5 K for 24 h, cooling at room temp.; SEM;In neat (no solvent); Mo powder reduction in H2 stream (850°C, 4 h), direct synthesis;In neat (no solvent); (vac.), heating (400°C, 2 d), heating (750°C, 48 h), heating (1000°C, 40 h), slow cooling;In neat (no solvent); react. Se with Mo (2:1) in evacuated (ca. 1E-2 Pa Ar) silica tube heatedat 700°C for 2 days;In neat (no solvent); multilayer deposition of vapors of Mo (from electron gun source) and Se (from Knudsen cell) onto Si-wafer at 5E-6 Torr, annealing at 550 K; 30-53% Mo in deposit, varying layer thickness; powder X-ray diffraction; Kinetics;In neat (no solvent); (Ar), glove-box; stoich. mixt. heated in evacuated silica tubes for ca. 2 d;In neat (no solvent, solid phase); heating of stoich. mixt. of elements in sealed evacuated silica tubes for 2 d at 1073 K;With dicopper(I) triselenostannate(IV); In neat (no solvent); at 550 ℃; Reagent/catalyst; Inert atmosphere;at 800 ℃; for 48h;at 699.84 ℃; for 48h; under 7.50075E-05 Torr; Sealed tube; Inert atmosphere;at 699.84 ℃; for 48h;In neat (no solvent, solid phase); at 799.84 ℃; for 12h; Sealed tube;at 600 ℃; for 48h; Sealed tube;at 600 ℃; for 48h; Sealed tube;-

- 7782-49-2
selenium

-
- 7631-95-0
sodium molybdate dihydrate

-

- 12058-18-3
molybdenum selenide
ConditionsConditions Yield With sodium tetrahydroborate; In water; at 200 ℃; for 12h; Autoclave;With hydrazine hydrate; sodium hydroxide; In water; at 180 ℃; for 48h; pH=12; Autoclave; High pressure;selenium; With hydrazine hydrate; for 1h;sodium molybdate dihydrate; In N,N-dimethyl-formamide; at 200 ℃; for 24h; Autoclave; High pressure;at 500 ℃; for 4h; Inert atmosphere;With sodium tetrahydroborate; In ethanol; water; at 200 ℃; for 48h; Autoclave;selenium; sodium molybdate dihydrate; With sodium tetrahydroborate; ethanol; In water; for 0.0833333h;In water; at 200 ℃; for 48h; Autoclave;at 450 ℃; for 3h; Inert atmosphere; Calcination;selenium; sodium molybdate dihydrate; With sodium tetrahydroborate; In water; at 20 ℃; for 0.833333h;In water; at 180 ℃; for 12h; Autoclave;selenium; With hydrazine hydrate; at 20 ℃; for 0.5h;sodium molybdate dihydrate; With sodium tetrahydroborate; In ethanol; water; for 0.5h;In ethanol; water; at 180 ℃; for 48h; Autoclave;12058-18-3 Upstream products
-
7782-49-2

selenium
-
7439-98-7

molybdenum
-
7631-95-0

sodium molybdate dihydrate
-
7446-08-4
selenium(IV) oxide