- Molecular Formula: NiO
- CasNo.: 1313-99-1
- Melting point: 1960 °C
- Appearance: olive green crystals
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
Best Quality Reputable Factory Supply 99.99%-99.999% Nickel Oxide 1313-99-1 with Efficient Delivery
- Molecular Formula:NiO
- Molecular Weight:74.6894
- Appearance/Colour:olive green crystals
- Melting Point:1960 °C
- PSA:17.07000
- Density:6.67 g/mL at 25 °C(lit.)
- LogP:-0.11880
Nickelous oxide(Cas 1313-99-1) Usage
Preparation
Nickel (II) oxide is prepared by heating pure nickel powder with oxygen at a temperature above 400°C. In some commercial processes, green Nickel (II) oxide is made by heating a mixture of nickel powder and water in air at 1,000°C. Adding some Nickel (II) oxide to the above mixture enhances the rate of reaction. An alternative method of preparation of the green oxide involves thermal decomposition of an oxo acid salt of nickel at elevated temperatures. Thus, nickel nitrate, nickel sulfate or, more conveniently, nickel carbonate when heated at 1,000°C, yields the green oxide. The black oxide, on the other hand, is produced at a lower temperature from incomplete calcination of the carbonate or nitrate salt at 600°C. The oxygen content of the black form is slightly greater than its green counterpart.
Reactions
Several nickel salts are obtained by reactions of nickel oxide with mineral acids. Thus, the reaction of black nickel oxide with hot dilute sulfuric acid forms nickel sulfate, NiSO4?6H2O. Similarly, dilute nitric acid, hydrochloric, and hydrobromic acids when heated react with the black form of nickel oxide to yield corresponding nickel salts as hexahydrates. Heating nickel oxide with hydrogen, carbon, or carbon monoxide reduces it to metallic nickel. Nickel oxide combines with sodium or potassium hydroxide at elevated temperatures (>700°C), forming sodium or potassium nickelate; i.e., K2NiO2: NiO + 2NaOH → Na2NiO2 + H2O
Hazard
Confirmed carcinogen.
Flammability and Explosibility
Nonflammable
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by intratracheal, intravenous, and subcutaneous routes. Mutation data reported. Can react violently with fluorine, hydrogen peroxide, hydrogen sulfide, iodine, barium oxide + air. See also NICKEL COMPOUNDS.
Physical Properties
Green cubic crystals; transforms to a grayish black octahedral form, known as black oxide, when strongly ignited; black oxide has a metallic luster; density of green oxide is 6.72 g/cm3; Mohs hardness 5.5; melts at 1955°C; insoluble in water; soluble in acids at ordinary temperatures; black form dissolves in hot acids.
General Description
Nickel(II) oxide (NiO) is a metal oxide based nanomaterial with a good semiconducting property. Nanosized nickel oxide can be found in a variety of morphologies which include nanoflowers, spheres, wires, and tubes. It exhibits high performance in applications which require charge transfer and charge transport based processes. It can be prepared by a variety of physical and thermal methods such as sol-gel, hydrothermal and solvothermal techniques.
InChI:InChI=1/Ni.O/q+2;-2
1313-99-1 Relevant articles
Spectral, thermal, and X-ray studies on some new bis and tris-hydrazine and hydrazinium metal pyruvates
Raju,Sivasankar
, p. 371 - 376 (2009)
Some bis-hydrazine metal pyruvates of tr...
Electric field formation during combustion of single metal particles
Martirosyan,Filimonov,Nersesyan,Luss, Dan
, p. J9-J16 (2003)
A strong electric field formed during th...
K4Ni3O6 and KNa2Ni 2O4, new quasi one-dimensional oxonickelates(II, III)
D Strok Signuris, Katarina,Nuss, Juergen,Jansen, Martin
, p. 2755 - 2760 (2013)
Two new quasi one-dimensional alkali met...
Preparation and characterization of NiO nanoparticles from thermal decomposition of the [Ni(en)3](NO3)2 complex: A facile and low-temperature route
Farhadi, Saeid,Roostaei-Zaniyani, Zeinab
, p. 971 - 975 (2011)
NiO nanoparticles with an average size o...
Spin-gap formation and thermal structural studies in reduced hybrid layered vanadates
Yan, Bangbo,Luo, Junhua,Dube, Paul,Sefat, Athena S.,Greedan, John E.,Maggard, Paul A.
, p. 5109 - 5118 (2006)
Reduced layered M(C4H4N2)V 4O10 ((I, M =...
The combined effect of mechanical and thermal energy on the solid-state formation of NiFe2O4 from the system 2NiCO3·3Ni(OH)2·4H2O-FeC2O4·2H2O
Berbenni, Vittorio,Milanese, Chiara,Bruni, Giovanna,Marini, Amedeo
, p. 86 - 90 (2008)
Spinel-type ferrites MFe2O4 (M = Ni, Zn,...
Catalytic hydrocarboxylation of acetylene to acrylic acid using Ni2O3 and cupric bromide as combined catalysts
Lin, Tie Jun,Meng, Xuan,Shi, Li
, p. 77 - 83 (2015)
A non-petroleum route to produce acrylic...
Synthesis, characterization, corrosion inhibition of mild steel in HCl (0.5?N) solution and solid-state electrical conductivity of new Co(II), Ni(II), Cu(II) and Zn(II) complexes
Nassar,Hassan,Elkmash
, (2017)
This work consists of a study of the cor...
Investigation of porous ni-based metal-organic frameworks containing paddle-wheel type inorganic building units via high-throughput methods
Maniam, Palanikumar,Stock, Norbert
, p. 5085 - 5097 (2011)
In the search of Ni based metal-organic ...
Cs2NiO2 revisited. Crystal structure and magnetic properties
Duris, Katarina,Jansen, Martin
, p. 57 - 60 (2012)
Single crystals as well as microcrystall...
Thermolysis of Coprecipitated Copper(II)-Nickel(II) Hydroxides
Kopylovich,Kirillov,Baev
, p. 12 - 17 (2001)
Coprecipitated copper(II)-nickel(II) hyd...
Thermal and kinetic study of nickel trifluoromethanesulphonate, trifluoroacetate and acetate
De Souza,Sousa,Paiva,Borges,Melo,Scatena Jr.
, p. 959 - 962 (2008)
The reason of comparing thermal behaviou...
Synthesis and thermal decomposition of Zn(tda)H2O [tda = S(CH2COO)22-]
Wu, Ming-Cheng,Lee, Chi-Shen
, p. 9634 - 9636 (2006)
A novel two-dimensional coordination pol...
Thermal degradation of acetate-intercalated hydroxy double and layered hydroxy salts
Kandare, Everson,Hossenlopp, Jeanne M.
, p. 3766 - 3773 (2006)
Two hydroxy double salts (HDSs), zinc co...
Facile Synthesis of Hollow Polyhedral (Cubic, Octahedral and Dodecahedral) NiO with Enhanced Lithium Storage Capabilities
Lin, Feini,Wang, Hui,Wang, Gang
, p. 207 - 216 (2016)
A facile sacrificial-template method com...
49 Adsorption des Gaz par les Oxydes Pulverulents. I. Oxyde de Nickel
Teichner,Marcellini,Rué, Et P.
, p. 458 - 471 (1957)
L'oxyde de nickel est obtenu par dissoci...
Spectroscopic studies on Co(II), Ni(II), Cu(II) and Zn(II) complexes with a N4-macrocylic ligands
Swamy,Pola, Someshwar
, p. 929 - 933 (2008)
Complexes of cobalt(II), nickel(II), cop...
Simple and rapid synthesis of NiO/PPy thin films with improved electrochromic performance
Sonavane,Inamdar,Dalavi,Deshmukh,Patil
, p. 2344 - 2351 (2010)
Nickel oxide/polypyrrole (NiO/PPy) thin ...
Facile synthesis of nanocrystalline-assembled nest-like NiO hollow microspheres with superior lithium storage performance
Li, Yanwei,Zheng, Yuanyuan,Yao, Jinhuan,Xiao, Jianrong,Yang, Jianwen,Xiao, Shunhua
, p. 31287 - 31297 (2017)
Interconnected nest-like NiO hollow micr...
Synthesis, structure analysis and thermodynamics of [Ni(H 2O)4(TO)2](NO3)2· 2H2O (TO = 1,2,4-triazole-5-one)
Chen, San-Ping,Li, Na,Wei, Qing,Gao, Sheng-Li
, p. 1115 - 1120 (2010)
A novel complex [Ni(H2O)4(TO)2](NO3) 2· ...
The activities of some metal oxides in promoting the thermal decomposition of potassium oxalate
Mohamed, Mohamed A,Galwey, Andrew K,Halawy, Samih A
, p. 63 - 74 (2002)
Transition metal oxalates undergo anion ...
Oxygen non-stoichiometry and reducibility of B-site substituted lanthanum manganites
Patcas,Buciuman,Zsako
, p. 71 - 76 (2000)
LaMn0.8B'0.2O3+(δ) (B'=Ni, Zn, Cu) and L...
Some aspects of thermal decomposition of NiC2O4·2H2O
Ma?ecka,Ma?ecki,Drozdz-Cie?la,Tortet,Llewellyn,Rouquerol
, p. 57 - 62 (2007)
NiC2O4·2H2O decomposition in air and in ...
Synthesis, characterization, and catalytic performance of La1-xCexNi1-yZryO3 perovskite nanocatalysts in dry reforming of methane
Dezvareha, Parastoo,Aghabozorgb, Hamidreza,Hossaini Sadrc, Moayad,Zared, Karim
, p. 1469 - 1477 (2018)
La1-xCexNi1-yZryO3 perovskite nanocataly...
Syntheses, crystal structures, and properties of Five new transition metal molybdenum(VI) selenites and tellurites
Zhang, Su-Yun,Jiang, Hai-Long,Sun, Chuan-Fu,Mao, Jiang-Gao
, p. 11809 - 11820 (2009)
Five new transition metal molybdenum(VI)...
The synergistic influences of OH- concentration and electrolyte conductivity on the redox behavior of Ni (OH) 2 /NiOOH
Hu, Chi-Chang,Chang, Kuo-Hsin,Hsu, Tung-Yu
, p. F196-F200 (2008)
The synergistic influences of the OH- co...
Perovskites as Precursors for Ni/La2O3 Catalysts in the Dry Reforming of Methane: Synthesis by Constant pH Co-Precipitation, Reduction Mechanism and Effect of Ru-Doping
Kühl, Stefanie,Düdder, Hendrik,Girgsdies, Frank,K?hler, Kevin,Muhler, Martin,Behrens, Malte
, p. 1088 - 1095 (2017)
LaNiO3 perovskite is an interesting prec...
Morphological and pharmacological investigation on some biopotent materials derived from substituted pyrimidine and imidazole enzyme constituents
Shobana, Sutha,Subramaniam, Perumal,Dharmaraja, Jeyapraksh,Narayan, Arvind
, p. 242 - 253 (2014)
Coordinating behavior of novel N2O type ...
Nickel(II)?π interaction in [M(ampy)2Ni(μ-CN)2(CN)2]n (M = Zn(II) and Cd(II), ampy = 2-aminomethylpyridine): Syntheses, vibrational spectroscopy, thermal analyses and crystal structures of cyano-bridged heteronu
Kürk?üo?lu, Güne? Süheyla,Ye?ilel, Okan Zafer,Kavlak, Ilkan,Büyükgüng?r, Orhan
, p. 220 - 226 (2009)
Two novel three-dimensional cyano-bridge...
Unprecedented interweaving of hetero-chiral single helical chains into a 3D chiral framework with (10, 3) topology
Zhang, Cui-Qiao,Fu, Feng,Li, Dong-Sheng,Ren, Yi-Xia,Zhao, Xin-Ze
, p. 652 - 656 (2008)
The title compound, [Ni(dcbp)(H2O)2](H2d...
Exfoliation-free nanosheet synthesis of transition-metal hydroxynitrate and its transformation to oxide particulate nanosheet
Cui, Hongtao,Zayat, Marcos,Levy, David
, p. 144 - 145 (2007)
A new strategy, epoxide-assisted precipi...
Spectroscopic studies and thermal decomposition for (bis-((E)-2-(4-ethylphenylimino)-1,2-diphenylethanone) Schiff base and its Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes prepared by direct and template reactions
Emam, Sanaa M.,AbouEl-Enein, Saeyda A.,Emara, Esam M.
, p. 1611 - 1630 (2017)
A new Schiff base (bis-((E)-2-(4-ethylph...
Effect of operational key parameters on photocatalytic degradation of phenol using nano nickel oxide synthesized by sol-gel method
Hayat, Khizar,Gondal,Khaled, Mazen M.,Ahmed, Shakeel
, p. 64 - 71 (2011)
Photocatalytic oxidation of phenol was s...
Hydrothermal synthesis, crystal structures, and properties of CoII and NiII supramolecular complexes with 2,4,6-trimethyl benzoate and 4,4′-bipyridyl
Indrani, Murugan,Ramasubramanian, Ramasamy,Kumaresan, Sudalaiandi,Kang, Sung Kwon,Chen, Min,Du, Miao
, p. 3593 - 3600 (2008)
Two new coordination complexes, viz. [Co...
Preparation of nickel nanoparticles for catalytic applications
Morozov, Yu. G.,Belousova,Kuznetsov
, p. 36 - 40 (2011)
Spherical oxidized nickel particles 15 t...
Thermal, spectral and magnetic behaviour of 2,3,4-trimethoxybenzoates of Mn(II), Co(II), Ni(II) and Cu(II)
Ferenc, Wieslawa,Bocian, Beata,Sarzynski
, p. 377 - 383 (2006)
Four new complexes of 2,3,4-trimethoxybe...
Thermal studies of Co(II), Ni(II) and Cu(II) complexes of N,N′-bis(3,5-Di-t-butylsalicylidene)ethylenediamine
Dogan,Ulusoy,Oeztuerk,Kaya,Salih
, p. 267 - 276 (2009)
The thermal decomposition kinetics of st...
Kinetic and thermodynamics studies on the decompositions of Ni3C in different atmospheres
Leng, Yonghua,Xie, Lei,Liao, Fuhui,Zheng, Jie,Li, Xingguo
, p. 14 - 18 (2008)
The thermal decompositions (including TG...
Discharge-Induced Enhancement of the Oxygen Evolution Reaction
Ge, Baoxin,Hao, Jinhui,Hou, Jianwen,Li, Longhua,Luo, Wei,Shi, Weidong,Wang, Shuaishuai,Yang, Wenshu,Zhao, Kun
, p. 20042 - 20048 (2021)
The fundamental understanding of the sur...
A series of bis(pyridyl)-bis(amide)-modulated metal-1,2-phenylenediacetate coordination polymers: construction and selective dye adsorption
Wang, Xiuli,Zhao, Jing,Le, Mao,Lin, Hongyan,Liu, Guocheng,Wang, Xiang
, p. 9316 - 9324 (2016)
Five new coordination polymers (CPs), na...
Self-assembled sandwich-like NiO film and its application for Li-ion batteries
Zhong,Wang,Xia,Gu,Xiang,Zhang,Tu
, p. 3889 - 3893 (2011)
NiO film with sandwich-like morphology i...
1D hydrogen-bonded infinite chains from tetraaza macrocycle nickel(II) complexes and ligands
Lim, In-Taek,Choi, Ki-Young
, p. 361 - 368 (2017)
The reaction of square planar complex [N...
The role of thermal analysis in optimization of the electrochromic effect of nickel oxide thin films, prepared by the sol-gel method: Part II
Cerc Koro?ec,Bukovec
, p. 65 - 71 (2004)
Thin films and the corresponding xerogel...
Nb effect in the nickel oxide-catalyzed low-temperature oxidative dehydrogenation of ethane
Zhu, Haibo,Ould-Chikh, Samy,Anjum, Dalaver H.,Sun, Miao,Biausque, Gregory,Basset, Jean-Marie,Caps, Valerie
, p. 292 - 303 (2012)
A method for the preparation of NiO and ...
Porous NiO/Ag composite film for electrochemical capacitor application
Wu,Li,Lin
, p. 2116 - 2121 (2011)
A highly porous NiO/Ag composite film is...
HSAB principle and nickel(II) ion reactivity towards 1-methyhydantoin
Puszyńska-Tuszkanow, Mariola,Daszkiewicz, Marek,Maciejewska, Gabriela,Staszak, Zbigniew,Wietrzyk, Joanna,Filip, Beata,Cie?lak-Golonka, Maria
, p. 2016 - 2025 (2011)
1-Methylhydantoin and its novel nickel(I...
Charge transporting enhancement of NiO photocathodes for p-type dye-sensitized solar cells
Hsu, Chih-Yu,Chen, Wei-Ting,Chen, Yung-Chung,Wei, Hung-Yu,Yen, Yung-Sheng,Huang, Kuan-Chieh,Ho, Kuo-Chuan,Chu, Chih-Wei,Lin, Jiann T.
, p. 210 - 215 (2012)
A p-type NiO film was prepared by doctor...
Interfacial Electron Redistribution of Hydrangea-like NiO@Ni2P Heterogeneous Microspheres with Dual-Phase Synergy for High-Performance Lithium–Oxygen Battery
Li, Runjing,Ran, Zhiqun,Shu, Chaozhu,Wen, Xiaojuan,Xu, HaoYang,Yan, Yu,Zeng, Ting,Zhao, Chuan
, (2022/01/19)
Lithium–oxygen batteries (LOBs) with ult...
Synthesis, characterization, in vitro antimicrobial and cytotoxic evaluation of Co(II), Ni(II), Cu(II) and Zn(II) complexes derived from bidentate hydrazones
Devi, Jai,Kumar, Sanjeev,Kumar, Deepak,Jindal, Deepak Kumar,Poornachandra
, p. 423 - 455 (2021/11/03)
Co(II), Ni(II), Cu(II) and Zn(II) comple...
Structural characterization, spectroscopic studies, and molecular docking studies on metal complexes of new hexadentate cyclic peptide ligand
Moustafa, Gaber,Sabry, Eman,Zayed, Ehab M.,Mohamed, Gehad G.
, (2021/12/08)
New cyclic peptide ligand (L), 4,7,17,20...
Antibacterial and anticorrosion behavior of bioactive complexes of selected transition metal ions with new 2-acetylpyridine Schiff base
Ashmawy, Ashraf M.,Deghadi, Reem G.,Elsharkawy, Ahmed E.,Mohamed, Gehad G.
, (2022/01/19)
Successful preparation of Schiff base 4-...
1313-99-1 Process route
-
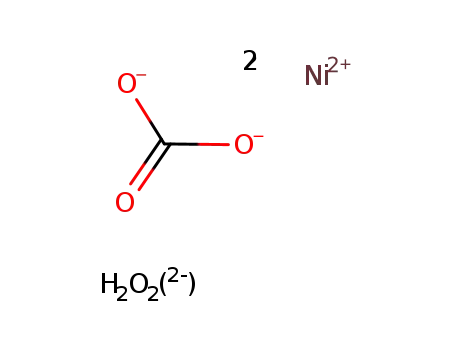
-
nickel hydroxide carbonate

-
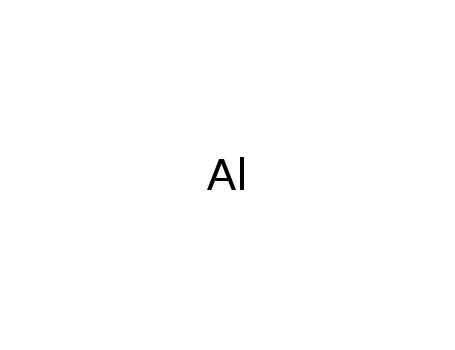
- 7429-90-5
aluminium

-

- 1313-99-1
nickel(II) oxide

-
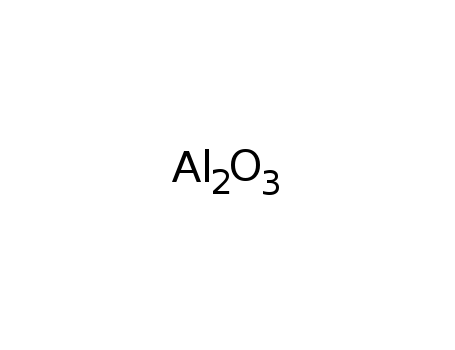
- 1333-84-2,1344-28-1
aluminum oxide

-
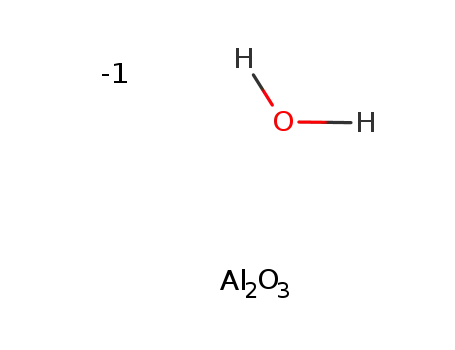
-
aluminium oxide hydrate
ConditionsConditions Yield In solid; byproducts: aluminium nickel alloy; ball milling of a mixt of Al and nickel salt in air; X-ray diffraction;-
-
4Ni(2+)*2Zn(2+)*2Al(3+)*16OH(1-)*CO3(2-)*4H2O=Ni4Zn2Al2(OH)16CO3*4H2O

-

- 1313-99-1
nickel(II) oxide

-

- 1333-84-2,1344-28-1
aluminum oxide

-
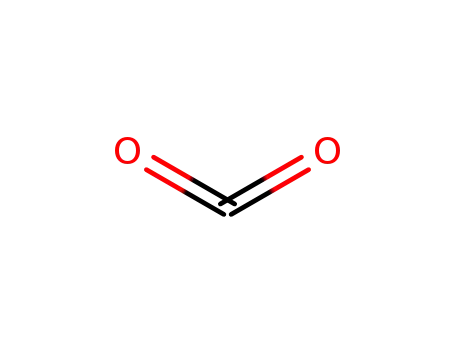
- 124-38-9,18923-20-1
carbon dioxide

-
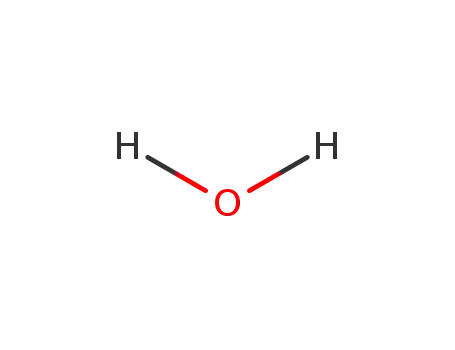
- 7732-18-5
water

-

-
zinc(II) oxide
ConditionsConditions Yield In neat (no solvent); thermal decomposition under N2; TG, DTG, MS;1313-99-1 Upstream products
-
6108-17-4
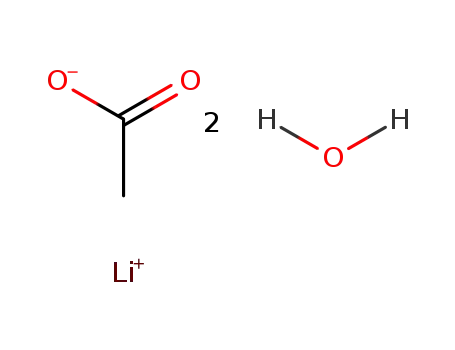
lithium acetate dihydrate
-
6018-89-9
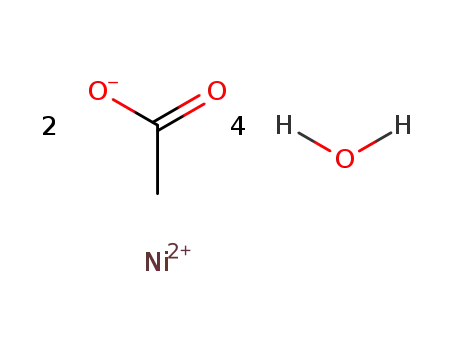
nickel(II) acetate tetrahydrate
-
7429-90-5

aluminium
-
1310-73-2

sodium hydroxide
1313-99-1 Downstream products
-
77-99-6
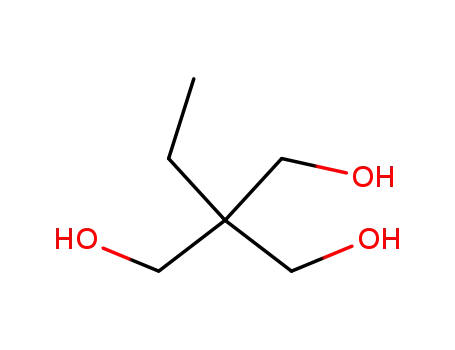
1,1,1-tri(hydroxymethyl)propane
-
83864-14-6
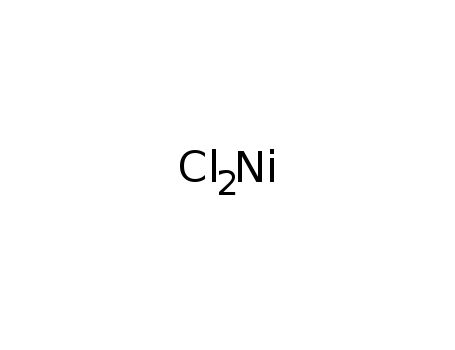
nickel dichloride
-
7440-02-0

nickel
-
13463-39-3
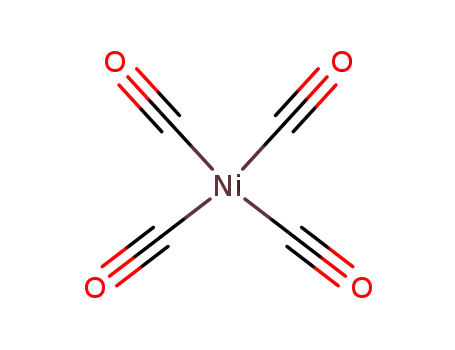
tetracarbonyl nickel

