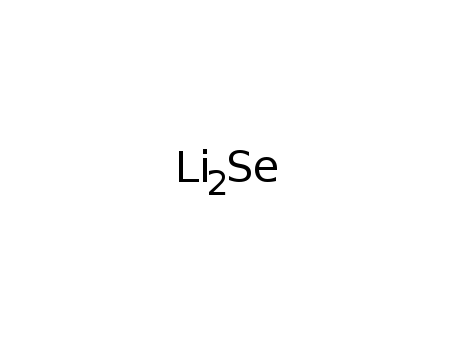- Molecular Formula: Li2Se
- CasNo.: 12136-60-6
- Melting point: 1302 °C
- Appearance:
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
Reliable Quality Trustworthy Manufacturer Supply 99.9% Lithium Selenide 12136-60-6 with Efficient Shipping
- Molecular Formula:Li2Se
- Molecular Weight:92.842
- Melting Point:1302 °C
- Boiling Point:°Cat760mmHg
- Flash Point:°C
- PSA:0.00000
- Density:g/cm3
- LogP:-0.38080
LITHIUM SELENIDE(Cas 12136-60-6) Usage
General Description
Lithium Selenide (Li2Se) is a chemical compound composed of lithium, an alkali metal, and selenium, a nonmetal. It appears as a white or off-white solid substance. LITHIUM SELENIDE is highly reactive due to its active ingredients and has a molecular weight of 133.83 g/mol. It has potential uses in various applications, notably in the field of optics and lithium-ion batteries. However, due to its corrosive and toxic nature, precautions must be taken when handling or storing Lithium Selenide, by ensuring adequate ventilation and minimizing human contact.
InChI:InChI=1/2Li.Se/rLi2Se/c1-3-2
12136-60-6 Relevant articles
Li7Cd4.5Ge4Se16 and Li6.4Cd4.8Sn4Se16: Strong Nonlinear Optical Response in Quaternary Diamond-Like Selenide Networks
Guo, Yangwu,Li, Xiaoshuang,Feng, Kai,Li, Chao,Zhou, Molin,Wu, Yicheng,Yao, Jiyong
, p. 871 - 876 (2018)
Two new selenides with diamond-like stru...
Organosilicon chalcogenides with trisilane units - Bicyclo[3.3.1]nonanes, bicyclo[3.2.2]nonanes and spiro[4.4]nonanes
Herzog, Uwe,Borrmann
, p. 564 - 574 (2004)
Treatment of 1,2,3-trichloropentamethylt...
Two-Dimensional Substitution: Toward a Better Understanding of the Structure-Transport Correlations in the Li-Superionic Thio-LISICONs
Minafra, Nicolò,Hogrefe, Katharina,Barbon, Federico,Helm, Bianca,Li, Cheng,Wilkening, H. Martin R.,Zeier, Wolfgang G.
, p. 727 - 740 (2021)
A deeper understanding of the relationsh...
Room-temperature synthesis, hydrothermal recrystallization, and properties of metastable stoichiometric FeSe
Nitsche,Goltz,Klauss,Isaeva,Mueller,Schnelle,Simon,Doert, Th.,Ruck
, p. 7370 - 7376 (2012)
Room-temperature precipitation from aque...
Cyclic Silylselenides: Convenient Selenium Precursors for Atomic Layer Deposition
Charvot, Jaroslav,Pokorny, Daniel,Zazpe, Raul,Krumpolec, Richard,Pavliňák, David,Hromádko, Luděk,P?ikryl, Jan,Rodriguez-Pereira, Jhonatan,Klikar, Milan,Jelínková, Veronika,Macak, Jan M.,Bure?, Filip
, p. 576 - 579 (2020)
Three cyclic silylselenides were prepare...
Deposition of MoSe2flakes using cyclic selenides
Bure?, Filip,Charvot, Jaroslav,Jelínková, Veronika,Klikar, Milan,Krumpolec, Richard,Macak, Jan M.,Pavliňák, David,Pokorny, Daniel,Rodriguez-Pereira, Jhonatan,Zazpe, Raul
, p. 22140 - 22147 (2021)
The currently limited portfolio of volat...
Direct thermal neutron detection by the 2D semiconductor 6LiInP2Se6
Chica, Daniel G.,He, Yihui,McCall, Kyle M.,Chung, Duck Young,Pak, Rahmi O.,Trimarchi, Giancarlo,Liu, Zhifu,De Lurgio, Patrick M.,Wessels, Bruce W.,Kanatzidis, Mercouri G.
, p. 346 - 349 (2020)
Highly efficient neutron detectors are c...
Soluble semiconductors AAsSe2 (A = Li, Na) with a direct-band-gap and strong second harmonic generation: A Combined experimental and theoretical study
Bera, Tarun K.,Jang, Joon I.,Song, Jung-Hwan,Malliakas, Christos D.,Freeman, Arthur J.,Ketterson, John B.,Kanatzidis, Mercouh G.
, p. 3484 - 3495 (2010)
AAsSe2 (A = Li, Na) have been identified...
Extended Chemical Flexibility of Cubic Anti-Perovskite Lithium Battery Cathode Materials
Lai, Kwing To,Antonyshyn, Iryna,Prots, Yurii,Valldor, Martin
, p. 13296 - 13299 (2018)
Novel bichalcogenides with the general c...
Synthesis, Structure and Application of Intramolecularly-Coordinated Gallium Chalcogenides: Suitable Single-Source precursors for GaxSey Materials
?i?ica, Tomá?,Bene?, Ludvík,Bou?ka, Marek,Dostál, Libor,Jambor, Roman,Knotek, Petr,Macak, Jan M.,Němec, Petr,R??i?ková, Zdenka,Ruleová, Pavlína
, p. 14470 - 14476 (2018)
Studies have been focused on the synthes...
Synthesis, crystal structure and lithium motion of Li8SeN 2 and Li8TeN2
Braeunling, Daniel,Pecher, Oliver,Trots, Dmytro M.,Senyshyn, Anatoliy,Zherebtsov, Dmitry A.,Haarmann, Frank,Niewa, Rainer
, p. 936 - 946 (2010)
The compounds Li8EN2 with E = Se, Te wer...
LiGaGe2Se6: A new IR nonlinear optical material with low melting point
Mei, Dajiang,Yin, Wenlong,Feng, Kai,Lin, Zheshuai,Bai, Lei,Yao, Jiyong,Wu, Yicheng
, p. 1035 - 1040 (2012)
The new compound LiGaGe2Se6 has been syn...
Oxidative Addition to SnII Guanidinate Complexes: Precursors to Tin(II) Chalcogenide Nanocrystals
Ahmet, Ibrahim Y.,Thompson, Joseph R.,Johnson, Andrew L.
, p. 1670 - 1678 (2018)
SnS, SnSe and SnTe are potentially impor...
Li6+2x[B10Se18]Sex (x ≈ 2), an ion-conducting double salt
Hammerschmidt,Doech,Puetz,Eckert,Krebs
, p. 1219 - 1226 (2006)
Li6+2x[B10Se18]Sex (x ≈ 2) was prepared ...
Syntheses and characterization of some mixed Te/Se polychalcogenide anions [TemSen]2-
Sekar, Perumal,Ibers, James A.
, p. 5436 - 5441 (2004)
Several mixed Te/Se polychalcogenide ani...
(De)lithiation mechanism of Li/SeSx (x = 0-7) batteries determined by in situ synchrotron x-ray diffraction and X-ray absorption spectroscopy
Cui, Yanjie,Abouimrane, Ali,Lu, Jun,Bolin, Trudy,Ren, Yang,Weng, Wei,Sun, Chengjun,Maroni, Victor A.,Heald, Steve M.,Amine, Khalil
, p. 8047 - 8056 (2013)
Electrical energy storage for transporta...
Structure Tuning, Strong Second Harmonic Generation Response, and High Optical Stability of the Polar Semiconductors Na1- xKxAs Q2
Iyer, Abishek K.,Cho, Jeong Bin,Byun, Hye Ryung,Waters, Michael J.,Hao, Shiqiang,Oxley, Benjamin M.,Gopalan, Venkat,Wolverton, Christopher,Rondinelli, James M.,Jang, Joon I.,Kanatzidis, Mercouri G.
, p. 18204 - 18215 (2021/11/12)
The mixed cation compounds Na1-xKxAsSe2 ...
Traceless selenocarboxylates for the one-pot synthesis of amides and derivatives
Silva, Luana,Rosário, Alisson R.,Machado, Bianca M.,Lüdtke, Diogo S.
supporting information, (2020/12/25)
We have recently reported a one-pot proc...
12136-60-6 Process route
-

- 7782-49-2
selenium

-
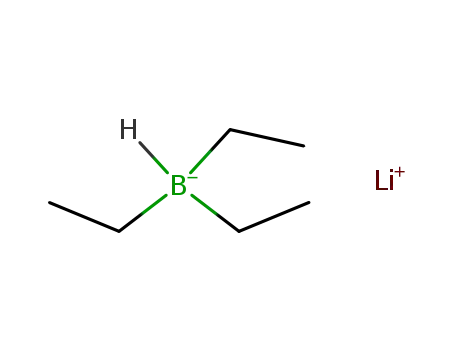
- 22560-16-3
lithium triethylborohydride

-
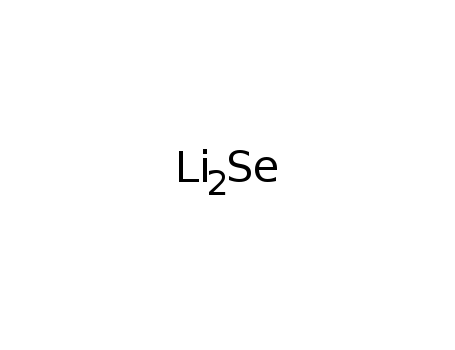
- 12136-60-6
lithium selenide
ConditionsConditions Yield In tetrahydrofuran; (Ar); addn. of selenium to a soln. of boron compd. in THF with stirring;In tetrahydrofuran; Se powder (1.5 mmol) was added to a soln. of B-contg. compd. in THF;In tetrahydrofuran; byproducts: B(C2H5)3, H2;In tetrahydrofuran;In tetrahydrofuran; at -20 ℃; Schlenk technique; Inert atmosphere;In tetrahydrofuran; Inert atmosphere; Schlenk technique;In tetrahydrofuran; at 25 ℃; for 2h;In tetrahydrofuran; at 20 ℃; for 4h; Inert atmosphere; Schlenk technique;In tetrahydrofuran; at 20 ℃; for 0.0833333h; Inert atmosphere;In tetrahydrofuran; at 0 - 25 ℃; for 2h; Schlenk technique;-

- 7782-49-2
selenium

-

- 7439-93-2
lithium

-
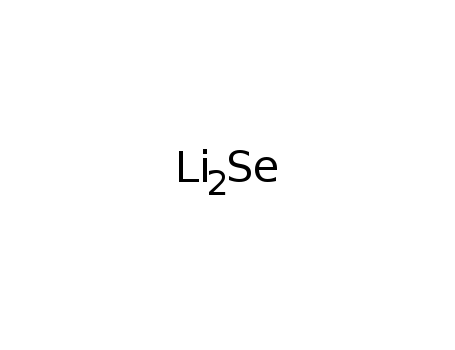
- 12136-60-6
lithium selenide
ConditionsConditions Yield In ammonia; addn. of Se to a soln. of Li in liq. NH3 at -33°C, formation of Li2Se;;In ammonia; -33°C, fast reaction;;In ammonia; NH3 (liquid); react. of Li and Se in liq. NH3;In ammonia; NH3 (liquid); addn. of Se to a soln. of Li in liq. NH3 at -33°C, formation of Li2Se;;In ammonia; NH3 (liquid); formation by reaction of Li with Se in liq. NH3, first precipitation of white amine, at 150°C in vac. formation of Li2Se;;In ammonia; NH3 (liquid); stoichiometric amount of Li and Se in liquid NH3;In not given; prepared from elements according to Thiele, K.-H. et al., Z. Anorg. Allg. Chem. 1996, 622, 231;In ammonia; NH3 (liquid);In ammonia; NH3 (liquid); stoich. amt. of Li and Se reacted in liq. NH3 at 194 K;In ammonia; NH3 (liquid); (N2, glovebox) stoichiometric amounts of elements in liquid NH3; Feher, F. Handbuch der Praeparative Anorganischen Chemie; Brauer, G., Ed.: Stuttgart, Germany, 1954, v.1, p 280-281; Aitken, J. A., Inorg. Chem. 2000, 39, 1525-1533;In melt; in sealed Ta ampoule at 575 K;In ammonia; aq. NH3; 194 K;With naphthalene; In tetrahydrofuran; (Ar); glovebox; Li and Se were reacted in THF in presence of naphthaleneas catalyst; washed (THF); dried (vac.);In ammonia;With ammonia;In ammonia; Inert atmosphere; Glovebox; Reflux; Cooling with acetone-dry ice; liquid NH3;at 399.84 ℃; for 48h; Inert atmosphere; Gas phase;In ammonia; liquid NH3;12136-60-6 Upstream products
-
7782-49-2

selenium
-
7439-93-2

lithium
-
12597-33-0
red selenium
-
22560-16-3

lithium triethylborohydride
12136-60-6 Downstream products
-
60763-24-8
ThSe2
-
105860-17-1
((C6H5)2SnSe)3
-
193338-96-4
2,4,6-tris[bis(trimethylsilyl)methyl]phenyl-1,3,5,2,4,6-triselenatribismane