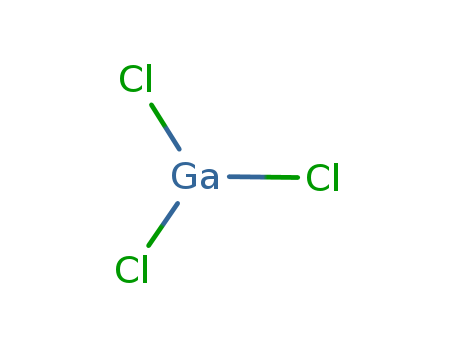
- Molecular Formula: GaCl3
- CasNo.: 13450-90-3
- Melting point: 78 °C(lit.)
- Appearance: white crystals or powder
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
Best Quality Quality Factory Supply 99.99%-99.999% Gallium chloride 13450-90-3 with Efficient Delivery
- Molecular Formula:GaCl3
- Molecular Weight:176.082
- Appearance/Colour:white crystals or powder
- Vapor Pressure:1 mm Hg ( 48 °C)
- Melting Point:78 °C(lit.)
- Boiling Point:35 °C
- Flash Point:-26 °F
- PSA:0.00000
- Density:2.47 g/mL at 25 °C(lit.)
- LogP:1.68770
GALLIUM(III) CHLORIDE(Cas 13450-90-3) Usage
Production Methods
The trichloride salt of trivalent gallium is prepared by the action of either hydrogen chloride or chlorine on the metal.
Air & Water Reactions
Water soluble.
Reactivity Profile
GALLIUM(III) CHLORIDE acts as a weak inorganic acid. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
(Non-Specific -- Gallium Compounds) In view of the toxicity of gallium and its compounds, as shown by experiments, all persons involved in work with these substances should undergo periodic medical examinations, during which special attention should be paid to the condition of the liver, respiratory organs, and skin.
Fire Hazard
Emits toxic chloride fumes when heated to decomposition. Decomposes upon sufficient heating.
Potential Exposure
Used as a raw material in the produc tion of metallic gallium; and in the processing of mono crystal, semiconductor compounds.
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required.
Purification Methods
The pure compound can be obtained by redistillation in a stream of Cl2 or Cl2/N2 followed by vacuum sublimation or zone refining. It forms colourless needles which give gallium dichloride [Ga(GaCl4), m 172.4o] on heating. It dissolves in H2O with liberation of heat. It is soluble in Et2O and can be extracted from an HCl solution with Et2O. [Laubengayer & Schirmer J Am Chem Soc 62 1579 1940, D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 846 1963.]
General Description
Colorless needles. Used as a raw material in the production of metallic gallium and in the processing of mono crystal semiconductor compounds.
InChI:InChI=1/3ClH.Ga/h3*1H;/q;;;+3/p-3
13450-90-3 Relevant articles
Thermogravimetric study of GaAs chlorination between -30 and 900 °c
Tunez, Fernando M.,Gonzalez, Jorge A.,Ruiz, María Del C.
, p. 124 - 136 (2011)
Gallium (as GaAs) is at present an essen...
Kinetics of the reaction of gallium arsenide with molecular chlorine
Ha, J. H.,Ogryzlo, E. A.,Polyhronopoulos, S.
, p. 2844 - 2847 (1988)
The reaction of Cl2 with the (100) face ...
The binary Ph2PCl/GaCl3 system: A room-temperature molten medium for P-P bond formation
Weigand, Jan J.,Burford, Neil,Decken, Andreas
, p. 4343 - 4347 (2008)
An equimolar reaction mixture of Ph2PCl ...
Thermodynamic characteristics of gaseous GaCl3pyz and GaCl 3pyzGaCl3 complexes
Timoshkin,Berezovskaya,Suvorov,Misharev
, p. 1173 - 1179 (2005)
Thermodynamic characteristics of vaporiz...
Gallium alkoxides: Synthesis and properties
Suslova,Turova,Mityaev,Kepman,Gohil
, p. 665 - 675 (2008)
Compounds Ga(OR)3 (R = Me, Et, Pri, Bun,...
Rhenium trichloride dioxide, ReO2Cl3, preparation and reactions
Supel, Joanna,Seppelt, Konrad
, p. 227 - 230 (2007)
A one step synthesis of ReO2Cl3 is repor...
Porous fluorinated aluminum and mixed gallium/aluminum oxide pillared tin phosphate materials with acid properties
Braos-Garci?a, Pilar,Rodri?guez-Castello?n, Enrique,Maireles-Torres, Pedro,Olivera-Pastor, Pascual,Jime?nez-Lo?pez, Antonio
, p. 1672 - 1678 (1998)
Fluorinated aluminum and mixed gallium/a...
GALLIUM DICHLORIDE. COMPOSITION OF SATURATED VAPOR. GEOMETRIC STRUCTURE AND VIBRATIONAL FREQUENCIES OF THE GaGaCl4 MOLECULE
Giricheva, N. I.,Girichev, G. V.,Titov, V. A.,Chusova, T. P.,Pavlova, G. Ya.
, (1992)
The saturated vapor above gallium dichlo...
Controlled synthesis of monodispersed AgGaS2 3D nanoflowers and the shape evolution from nanoflowers to colloids
Yuan, Yanping,Zai, Jiantao,Su, Yuezeng,Qian, Xuefeng
, p. 1227 - 1235 (2011)
Monodispersed AgGaS2 three-dimensional (...
Chemical and electrochemical behavior of gallium in the room-temperature ionic liquid of the composition [C6H11N 2][N(SO2CF3)2]
Smolenskii,Bove,Khokhryakov,Osipenko
, p. 499 - 502 (2003)
The behavior of gallium trichloride in t...
Activation of metallic aluminum by tin and gallium chlorides in oxidation with water
Burlakova,Shilkin,Kravchenko,Dremova,Kravchenko,Ivanov,Bulychev
, p. 238 - 243 (2012/07/02)
We have studied the effect of gallium ch...
The synthesis and deep purification of GaEt3. Reversible complexation of adducts MAlk3 (M = Al, Ga, In; Alk = Me, Et) with phenylphosphines
Shatunov,Korlyukov,Lebedev,Sheludyakov,Kozyrkin,Orlov, V.Yu.
, p. 2238 - 2251 (2011/06/22)
Optimal parameters of organomagnesium te...
13450-90-3 Process route
-
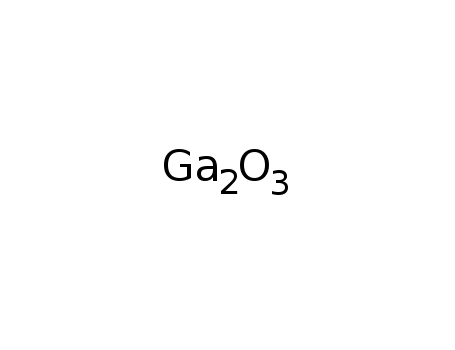
-
gallium(III) oxide

-
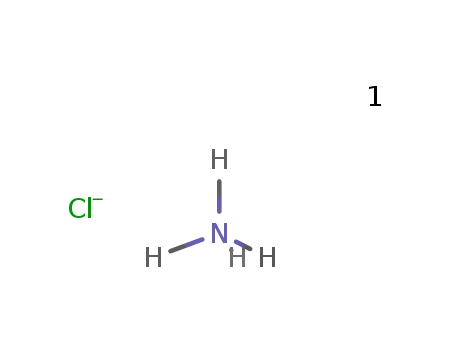
-
ammonium chloride

-
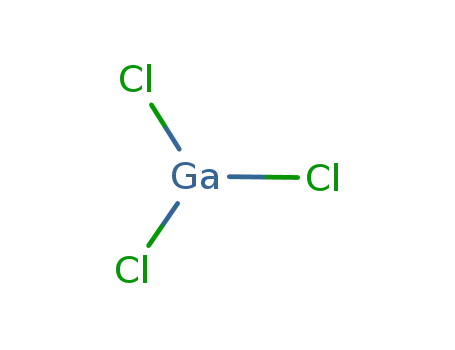
- 13450-90-3
Gallium trichloride
ConditionsConditions Yield In neat (no solvent); heating up to 250 °C;;In neat (no solvent); heating up to 250 °C;;-
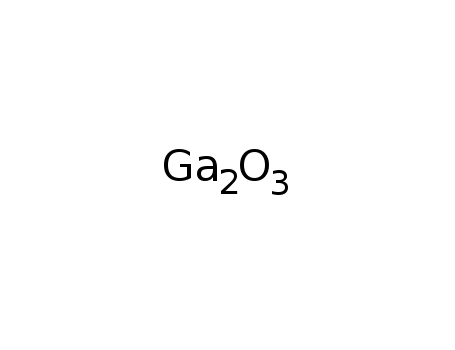
-
gallium(III) oxide

-
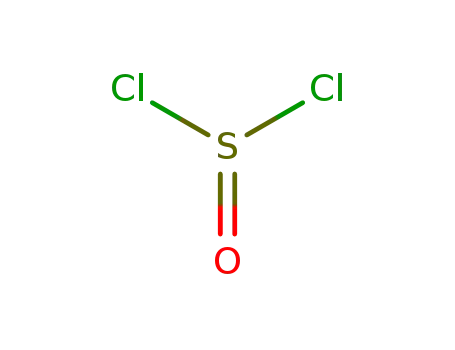
- 7719-09-7
thionyl chloride

-

- 13450-90-3
Gallium trichloride
ConditionsConditions Yield In neat (no solvent); 200°C;;In neat (no solvent); 200°C;;13450-90-3 Upstream products
-
7719-09-7

thionyl chloride
-
10026-04-7
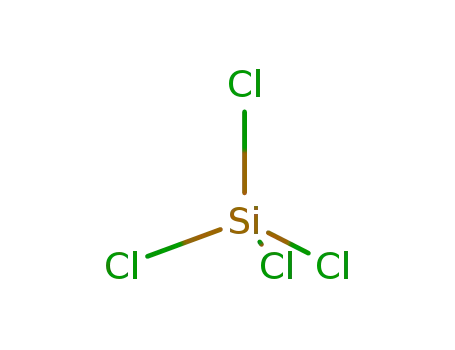
tetrachlorosilane
-
7440-55-3

gallium
-
7783-90-6
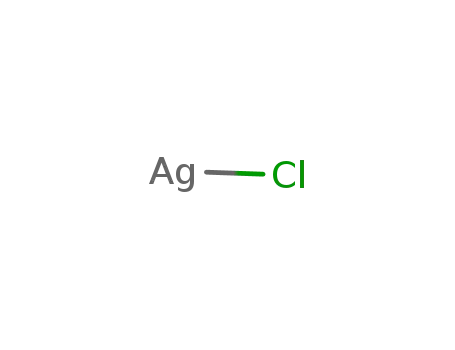
silver(I) chloride
13450-90-3 Downstream products
-
7440-55-3

gallium
-
1445-79-0
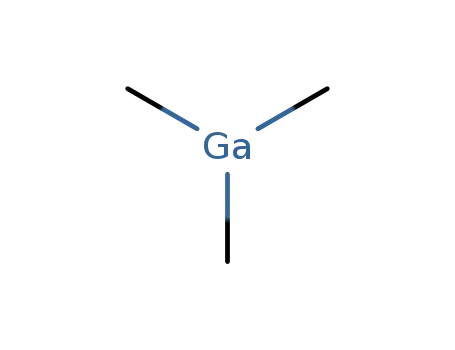
trimethyl gallium
-
126190-98-5
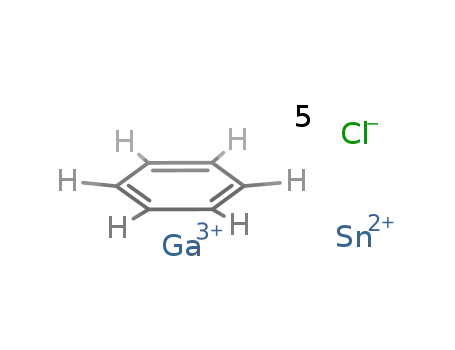
(benzene)chlorotin(+2) tetrachlorogallate(+3)
-
217479-82-8
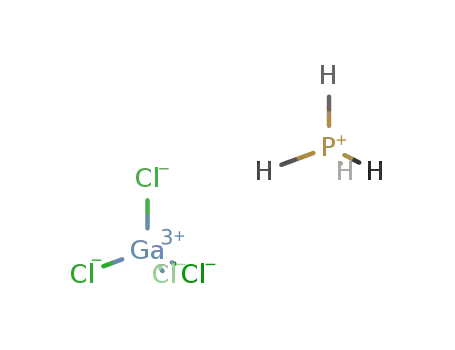
PH4(1+)*GaCl4(1-) = (PH4)(GaCl4)